FDA Approved Generic Propecia, Delivered To Your Door
115 reviews

More on finasteride
You can stop hair loss
Two-thirds of men will experience some degree of hair loss by age 35. If you decide to fight to keep it, we can help with a treatment plan and prescription (if appropriate).
How do I get generic propecia?
If appropriate, Roman-affiliated doctors and nurse practitioners can prescribe generic Propecia and send you a hair loss treatment plan designed by Roman’s physician advisors.
Is it delivered to my door?
Finasteride (generic Propecia) is an FDA-approved medication used to treat male pattern hair loss, also known as androgenetic alopecia. Finasteride slows or stops hair loss, and, in some cases, it may even regrow hair. Individual results will vary.
What is Propecia?
Propecia is the original brand name for 1 mg finasteride tablets for the treatment of male pattern hair loss.
How finasteride works
Finasteride is part of a family of medications called 5-alpha reductase inhibitors. These drugs work by blocking the activity of 5-alpha reductase, an enzyme that converts testosterone to dihydrotestosterone (DHT), a potent androgen (male sex hormone). DHT contributes to male pattern baldness (also known as androgenetic alopecia)but using 5-alpha reductase inhibitors to block this enzyme can slow or reverse hair loss.
What to expect
Our treatments are backed by real data and trusted by medical professionals.
1-2 Months: Save it
There will likely be no visible change in the first two months. Though some people may shed more fine hairs than before. This is temporary and normal.
3-6 Months: Regrow it
At this point, it may become noticeable that hair loss has slowed, stopped, or in some cases maybe even reversed. Take a photo to track your progress.
6 Months and Beyond
By now, hair loss may have considerably slowed or stopped, and some people may see signs of regrowth (usually at the crown of the head). Consistent daily treatment is required to maintain results and increase the chance of regrowth.
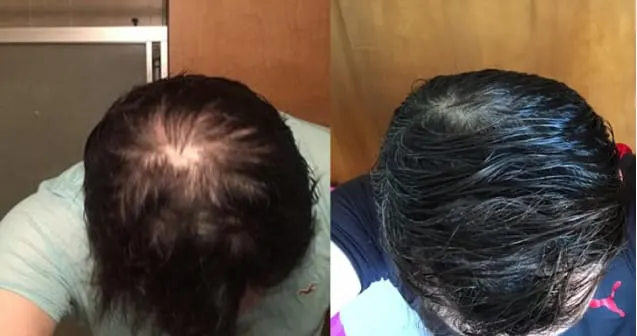
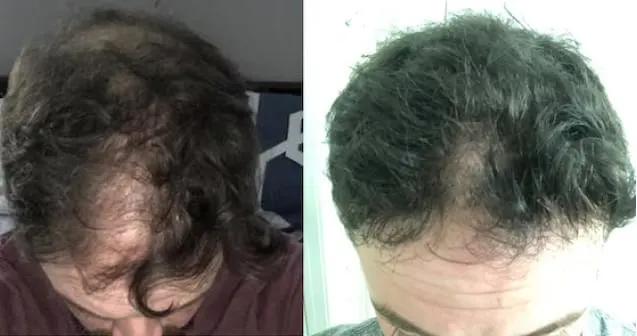
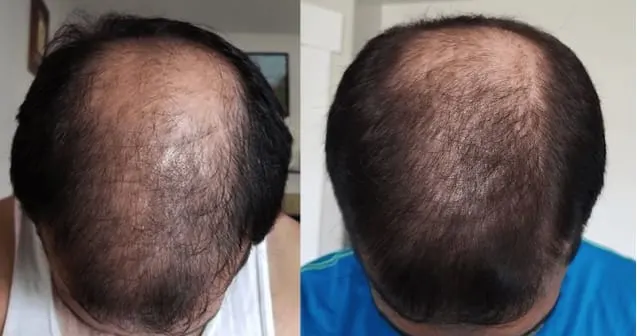
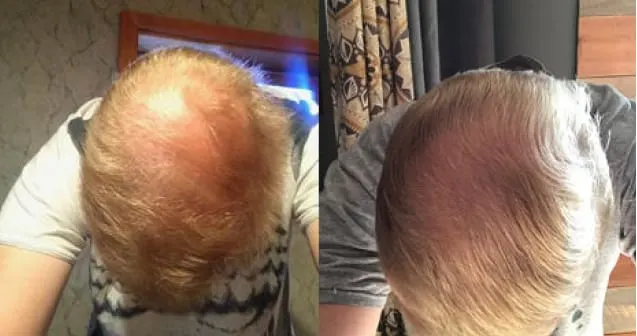
Real members. Real stories. Real results.
Before and After
Photos from Ro members taken after 6 months of treatment.
FAQ
How should I take finasteride?
Take finasteride exactly as your healthcare provider tells you to take it.
You may take finasteride with or without food.
If you forget to take finasteride, do not take an extra tablet. Just take the next tablet as usual.
Finasteride will not work faster or better if you take it more than once a day.
Can finasteride help with hair regrowth?
Short answer is yes. The same study from above that summarized the findings of 3 studies that evaluated the effects of finasteride showed that some men in the finasteride group has hair regrowth. The authors noted, “Based on standardized clinical photography, the chances of mild to moderate visible regrowth are 61% on the vertex (with an additional 5% achieving great visible regrowth) after 2 years and 37% on the frontal area after 1 year.” In the 2 studies done on hair loss at the crown of the head, 72% of men had further hair loss after 2 years. For clarity, vertex is the crown of the head and frontal area is the frontal or receding hairline.
What are the common side effects of finasteride?
The most common side effects of finasteride include:
decrease in sex drive
trouble getting or keeping an erection
a decrease in the amount of semen
What important safety information should I know before taking finasteride?
Please click here to see the full Important Safety Information.
Finasteride oral vs finasteride topical
Oral finasteride 1 mg is one of two FDA-approved treatments for male pattern hair loss, the other one being topical minoxidil 2% or 5%. Since oral finasteride has been shown to cause sexual side effects in a small number of men (3.8% of men who took finasteride vs 2.1% of men who took placebo in phase III clinical trials for finasteride), researchers have been interested in whether topical finasteride applied to the scalp may be effective in treating male pattern hair loss with a lower potential for side effects.
A 2018 systematic review of the results of seven different studies concluded that topical finasteride is effective for the treatment of male pattern hair loss but also lowers plasma (blood) DHT levels which can lead to undesired side effects like low libido. Still, it appears that the incidence of side effects was lower with topical finasteride than oral finasteride. Also, some researchers found that plasma DHT levels were only minimally decreased with topical finasteride.
At this time, topical finasteride is not FDA-approved for the treatment of male pattern hair loss and must be specially compounded by a compounding pharmacy and requires a doctor’s prescription. For this reason, compounded topical finasteride is not available through Roman.
Important safety information
What you should know before taking finasteride.