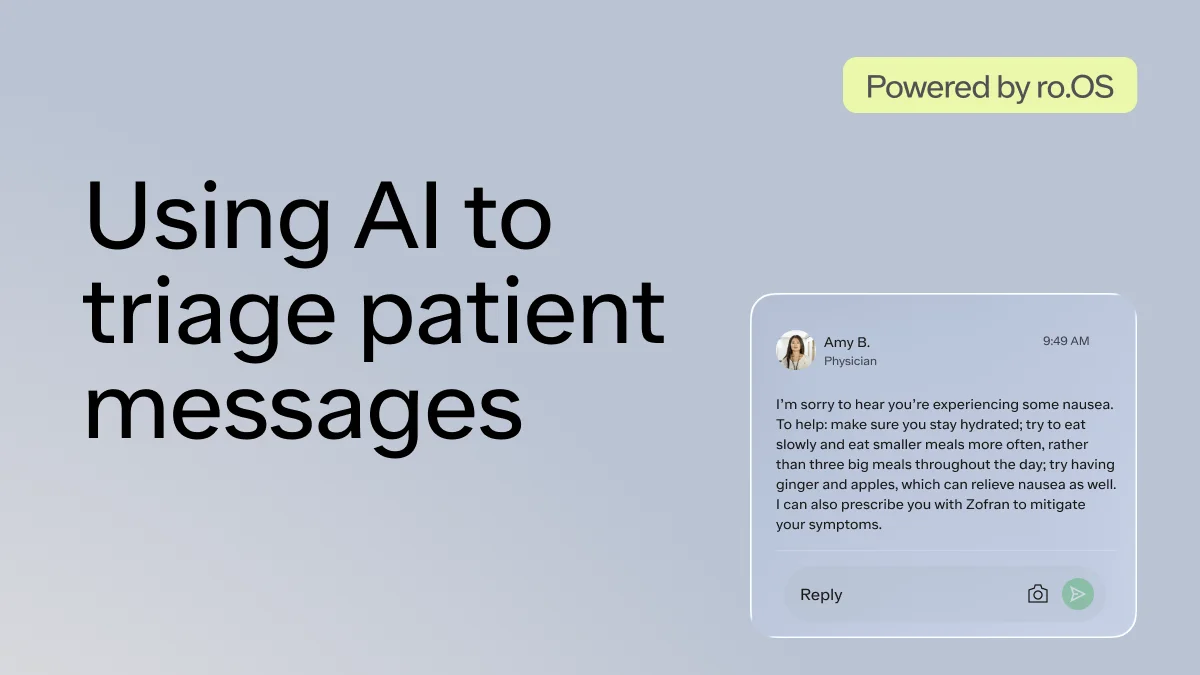
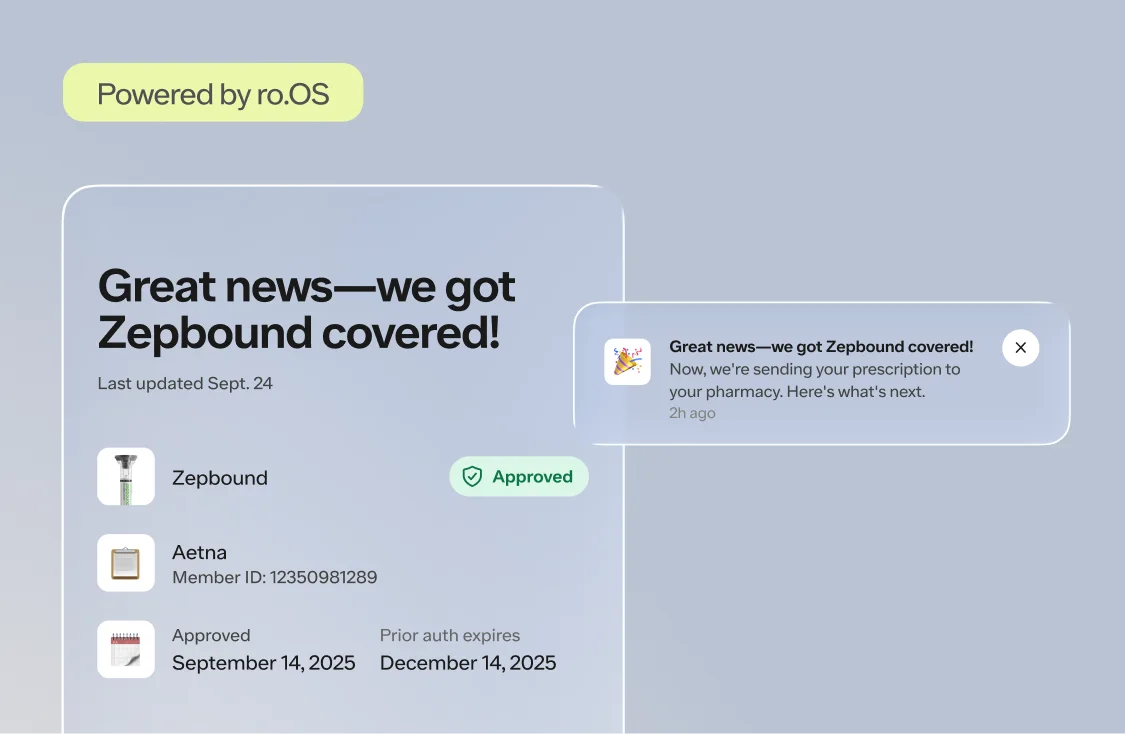
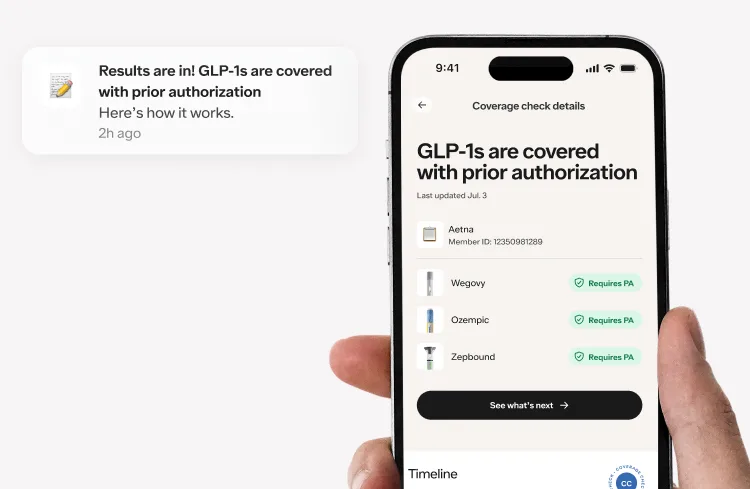
Here's what we'll cover
Here's what we'll cover
Here's what we'll cover
10/3/24 update: Analyzing September shortage submissions, 22% were for tirzepatide.
. . .
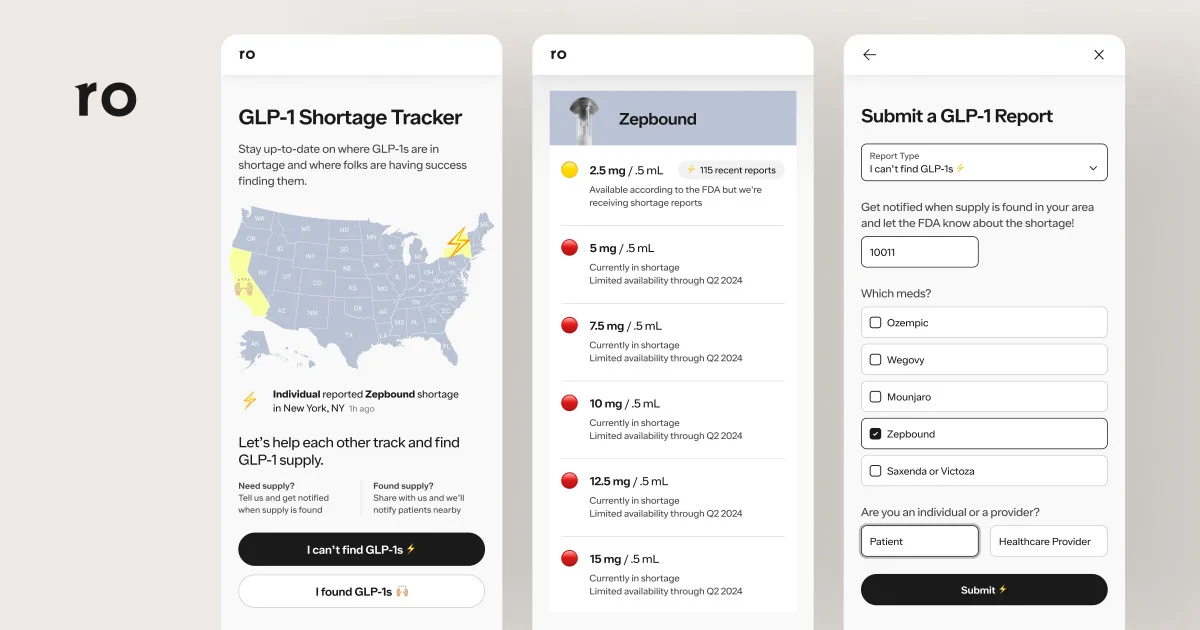
9/4/24 – Drug shortages continue to be a major hurdle preventing tens of millions of patients from starting or continuing their GLP-1 treatment. And the lack of accurate, up-to-date and easily accessible information on drug availability has made these shortages even harder to navigate.
That’s exactly why we launched the GLP-1 Supply Tracker. This free, open-access tool gives patients real-time insights on GLP-1 drug shortages and lets them sign-up for automated, localized supply alerts (more on how it works).
In the months since we launched the GLP-1 Supply Tracker, there’s been ongoing news and updates about the GLP-1 shortages. What matters most though is what patients are experiencing – are they able to reliably access their medication? Right now, the evidence suggests not.
What we know from patients
Since introducing our tool 3 months ago, patients have submitted over 60,000 shortage reports and we continue to receive thousands of reports each week.
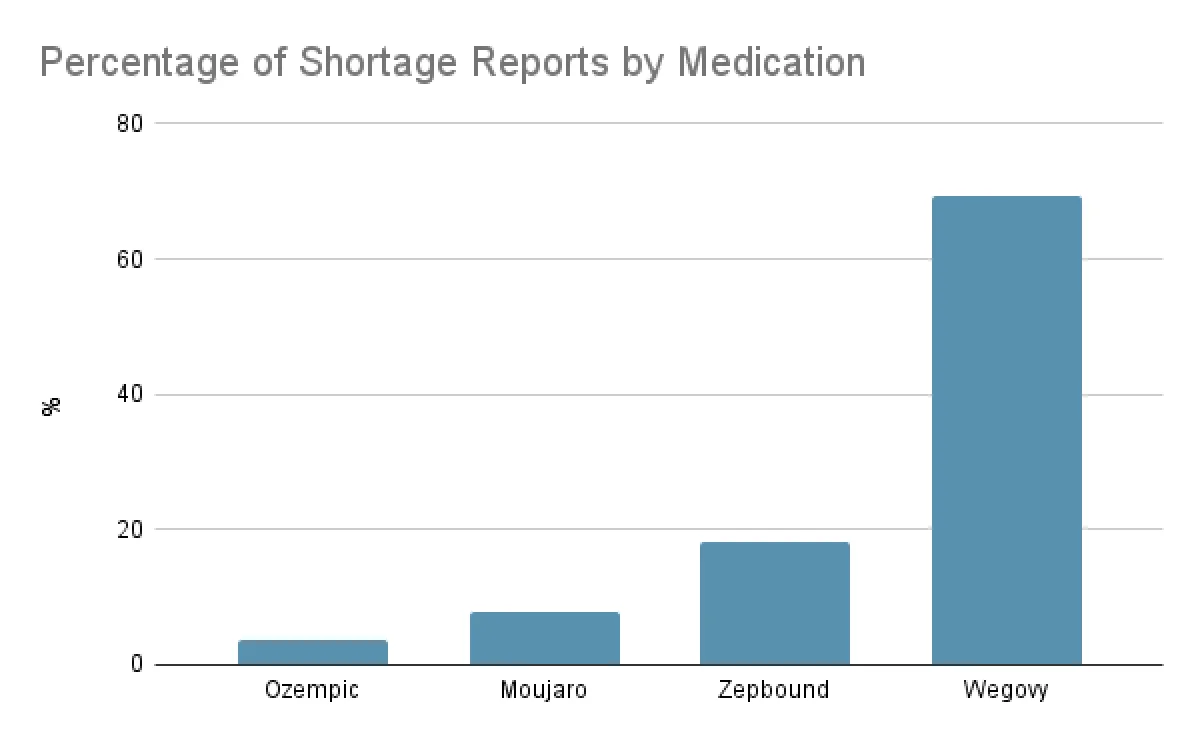
Wegovy and Zepbound are the most reported medications:
45% of all shortage reports are for Wegovy
34% of all shortage reports are for Zepbound
19% of all shortage reports are for Ozempic and Mounjaro, GLP-1s indicated for type-2 diabetes
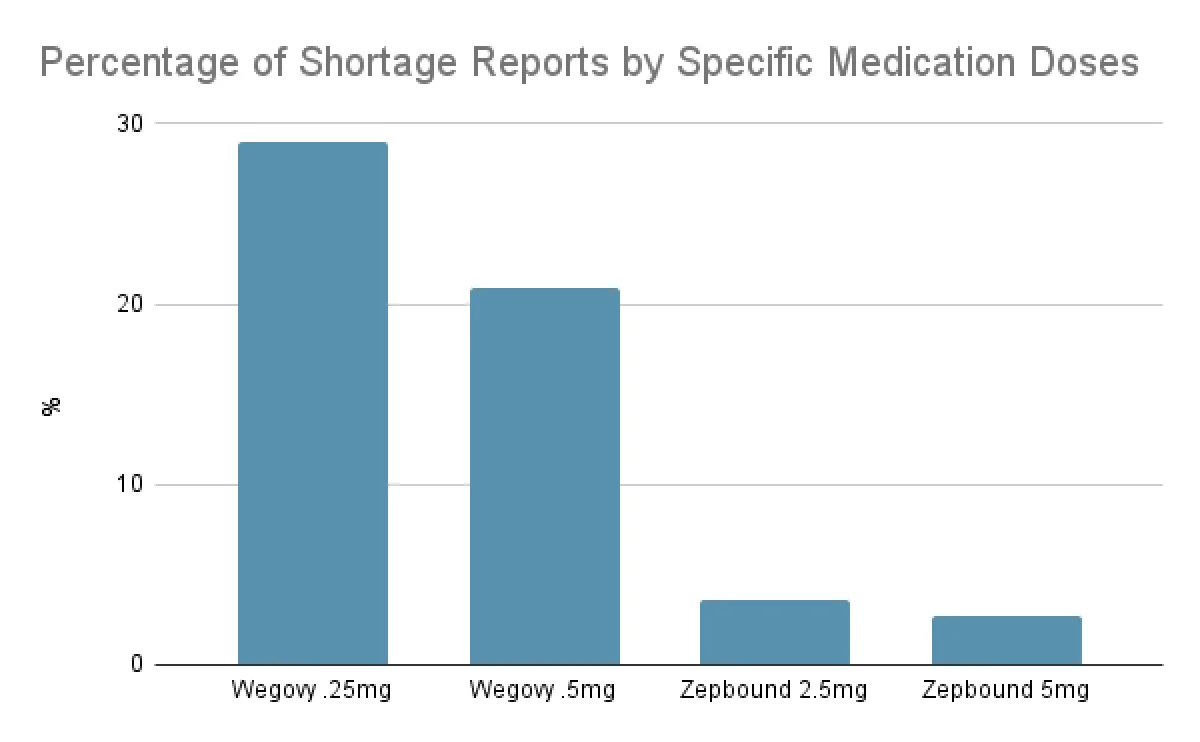
Starter doses are the most often reported:
Wegovy 0.25mg is the most commonly reported dosage, appearing in about twice as many reports as other Wegovy doses.
Zepbound 2.5mg is the most frequently reported dose of the drug.
Additionally, patients – whether Ro’s or those simply using our GLP-1 Supply Tracker to find their medication – have shared feedback about their difficulties finding supply of their medication. The quotes below are a sample of what we heard when automated alerts from our tracker notified patients about the FDA changing the availability status of certain Mounjaro and Zepbound doses in August. What’s clear from what we have heard from patients is that there is good reason for these drugs to still be on the shortage list. Even for doses marked ‘available’, patients are still not reliably accessing supply from their pharmacies.

Pharmacy supply insights
A drug wholesaler who Ro works with saw supply of GLP-1s begin to increase in July, but said that it remained difficult to maintain enough inventory to meet patient demand. The wholesaler shared that certain doses of medications continue to intermittently go out of stock.
Only one dose of Wegovy (2.4 mg), was consistently in stock over the past 8 weeks.
Wegovy 0.5mg was out of stock in 4 of the last 8 weeks.
The lower doses of Zepbound (2.5 mg and 5 mg) as well as the highest dose (15 mg) were consistently in stock over the past 8 weeks.
Zepbound 10 mg and 12 mg were out of stock in 4 of the last 8 weeks.
All doses of Ozempic were in stock throughout the past 8 weeks.
Three middle doses of Mounjaro (7.5 mg, 10 mg, and 12 mg) were out of stock at least one of the past 8 weeks.
Updates from pharmaceutical companies
Recently, Novo Nordisk and Eli Lilly have expressed increased belief in their ability to increase supplies of their GLP-1 medications, but have acknowledged that some patients will still experience shortages. Novo Nordisk noted during the company’s earnings call on August 7 that demand is still expected to surpass supply, but the company is continuing to focus on expanding supply so more patients can access its medications. Similarly, Eli Lilly highlighted on August 8 that progress on supply has bolstered their outlook, but that “the end pharmacy experience will continue to be choppy.”
The FDA’s Drug Shortage List
The GLP-1 shortage began in March 2022, with semaglutide injection (the active pharmaceutical ingredient in Ozempic and Wegovy) first appearing on the FDA’s shortage list, followed by tirzepatide injection (the active pharmaceutical ingredient in Mounjaro and Zepbound) in December 2022. In August, all Wegovy doses except for 0.25mg and all doses of Ozempic, Zepbound, and Mounjaro were marked “available” according to the FDA’s list. Importantly though, the FDA still lists both semaglutide and tirzepatide injections as “Currently in Shortage” – its designation that there is a drug shortage and the drug is not commercially available.
The agency recently explained that it considers the bigger picture in determining when a drug should be on its shortage list, saying, “FDA generally considers a shortage to be resolved … based on an evaluation of the entire market, assessing whether all backorders have been filled and supply is meeting or exceeding demand.”
Could things be improving?
While evidence demonstrates that drug shortages have been persistent and continue affecting patients, there are some signs of supply progress among some GLP-1s medications. In August, Zepbound only accounted for ~18% of all shortage reports to our GLP-1 Supply Tracker. Similarly, while the starter doses of Wegovy, 0.25mg and 0.5mg, accounted for 29% and 21% of shortage reports respectively in August, the lower doses of Zepbound, 2.5mg and 5mg, accounted for less than 4% and 3% respectively.
Navigating the shortage is hard, but finding GLP-1s shouldn’t be. Ro is proud that our tracker, and other free tools that we’ve built, such as the GLP-1 Insurance Checker, are helping patients navigate their GLP-1 journey. However, we know there is more to do. There are currently 5-10 million people on a branded GLP-1 medication, but there is an estimated 50-60 million who are clinically eligible to receive Wegovy and have insurance coverage for it, and of those, millions could purchase it for a copay of less than $100/month. That is a lot of unmet demand that supply still must meet.
At Ro, we are rooting for the shortages to end as quickly as possible so that every single patient who could benefit from a GLP-1 medication has full, uninterrupted access. In the meantime, we’ll continue to fight for patients every single day: helping ensure continuity of care for those already on GLP-1s and enabling new patients to start the most effective treatment available.