Here's what we'll cover
Here's what we'll cover
Here's what we'll cover
Introduction
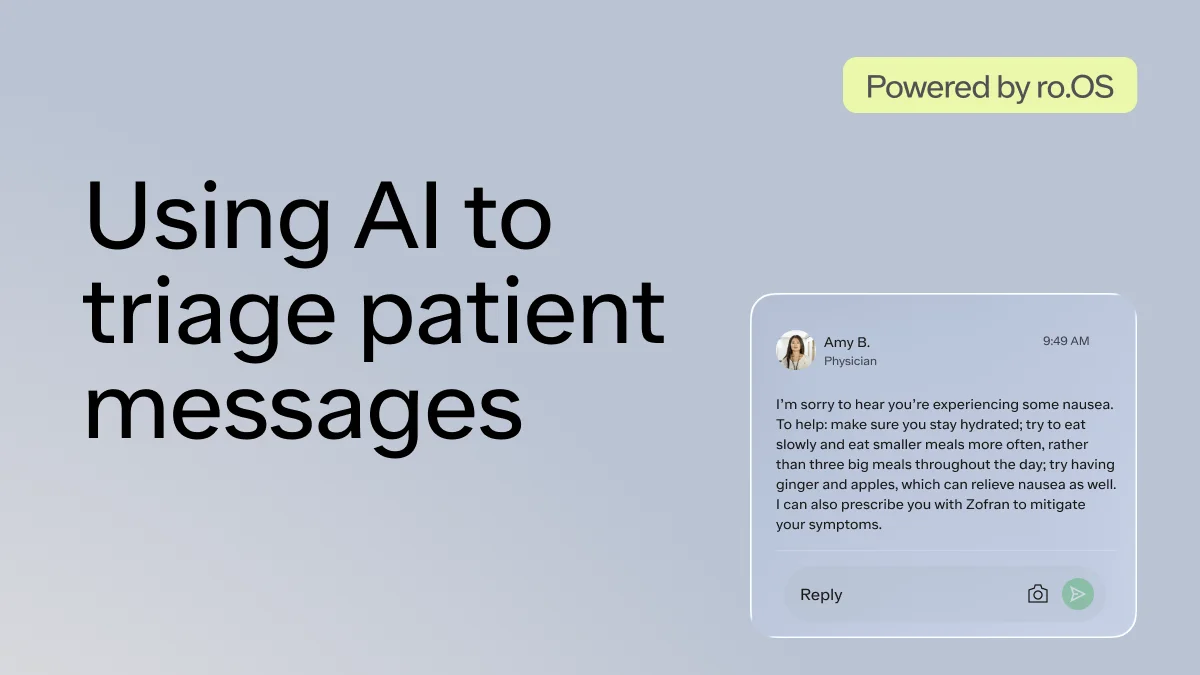
Over the last 3 years, Ro has helped hundreds of thousands of patients start their weight loss journey. We recently launched the Body Program, a comprehensive program to deliver high quality obesity care, centered around GLP-1s (e.g., semaglutide, Wegovy), with 1-1 provider guidance, 1-1 lifestyle and behavior coaching, lab testing, weight tracking, and 24/7 messaging.
By the time patients come to us, many have tried 4 or 5 different solutions and have struggled with their weight for 5-10 years, if not for the majority of their adult life. Some come to us expressing a level of hopelessness I’ve rarely seen. The pain they feel, their sense of failure, the seemingly insurmountable challenge of reaching their personal healthy weight, often leave them with a pervasive sense of failure that colors every aspect of their lives. For so many reasons, this should not be. Society's flawed judgements, the medical community's misplaced prioritization of generalized weight targets that are too strictly applied to individuals, and a food industry too little focused on health outcomes, subject people suffering from overweight and obesity to pressures that result in a sadness we have seen in few other conditions.
I originally created this Q&A for our team to help deepen our understanding of this disease and to improve how we can best support our patients. Each question came from a place of curiosity and a genuine desire to understand how we can best support those who experience overweight and obesity.
I decided to publish this as I’ve seen the public discourse talk about very similar subjects concerning weight loss and obesity, though often without a grounding in research and data. The reason I feel so strongly that this is important is because without a conversation grounded in data, I genuinely believe it will lead to increased suffering and fewer people getting access to the right care for them.
I’ve seen the impact of helping patients with obesity in large numbers and, personally, I saw it change my dad’s quality (and, hopefully, quantity) of life as I have never seen.
Talking about obesity is challenging. It’s infinitely nuanced clinically. It’s influenced by genetics, environment, and behavior. It’s also deeply personal. It’s influenced by our cultures, family, commerce, politics, philosophy, ethical considerations, sustainability beliefs, and on and on.
My hope is this post can help:
Answer some of the most common questions people might have about obesity and GLP-1s
Ground the conversation in more data and less opinion
Reduce even one person’s bias (conscious or subconscious) towards people with obesity
I know you might think this post is from just another startup founder in support of their own solution. I am amazed at the potential of GLP1s, but neither GLP1s nor Ro’s solution is right for everyone. My ultimate hope with this post is that, if nothing else, you see people who care deeply and are sincere in their pursuit to help people the best way they can.
I want to thank Dr. Tzvi Doron, Dr. Melynda Barnes, Dr. Raoul Manalac — for teaching me, putting up with me, and reviewing this post. I’d also like to thank our partners at the Obesity Action Coalition -- Joe Nadglowski and James Zervios -- for their insightful feedback.
Let’s dive in.
Why is obesity such an important problem to solve for patients?
Three reasons: health implications (physical, emotional, and psychological), prevalence, and cost.
Health Implications:
Obesity is a disease. Yes, a disease. The American Medical Association classified obesity as a disease in 2013 to aid in the research, prevention, and treatment of obesity as well as to reduce stigma and discrimination, which are exceedingly harmful and all too prevalent.
Unfortunately, a patient with obesity is often headed for a life with worse healthcare outcomes and greater healthcare costs. It is a contributor to 8 out of the top 10 leading causes of death in the U.S. Obesity is a risk factor for many diseases, including atherosclerotic vascular disease (causing heart attacks and strokes), type 2 diabetes, arthritis, several types of cancer, and premature death. Obesity also increases the risk of mental health disorders, including anxiety and depression.
We’ll get into much more detail on this data later in the post.
Prevalence:
Obesity has been steadily increasing from the mid 1970s and is the most common chronic disease in the U.S.
In 1990, zero states had obesity rates greater than 20%. By 2018, zero states had obesity rates less than 20%. (This is measured by BMI, more on the complexities of BMI below.)
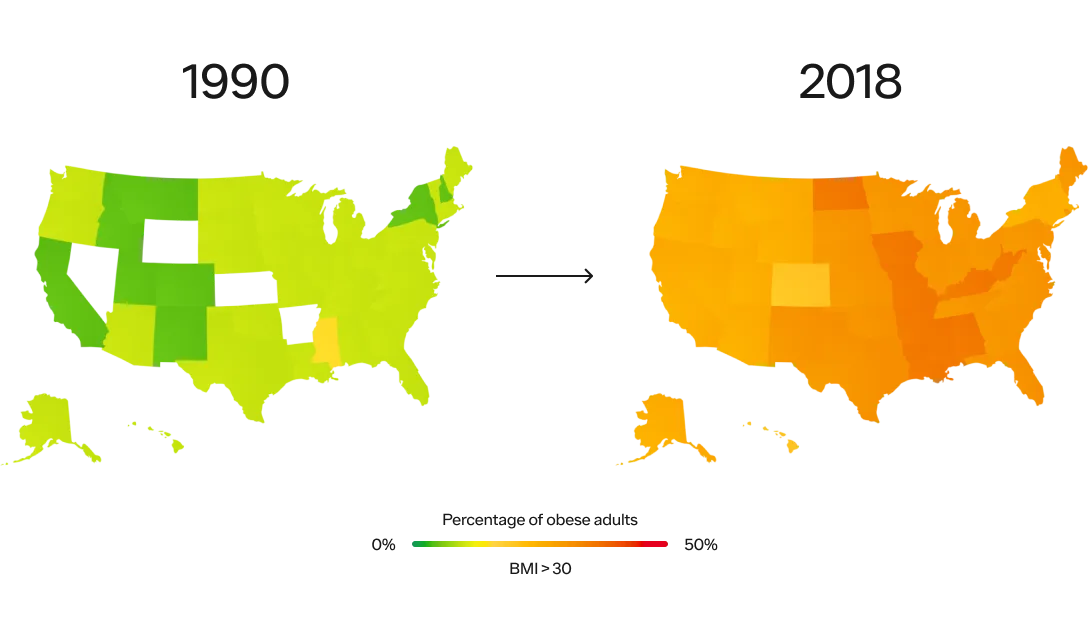
SOURCE: THE STATE OF OBESITY: BETTER POLICIES FOR AMERICA 2022
Obesity rates are continuing to climb. 74% of the U.S. population has overweight or obesity.
According to the CDC’s latest statistics, 42% of adults and 20% of children and adolescents have obesity as measured by BMI. Minorities, including Black and Hispanic people, are more affected than their white counterparts. These numbers don’t include the 31% of adults and 17% of children and adolescents with overweight. What’s worse, the proportion of the population with severe obesity has been rapidly increasing, with 9.2% of adults and 6.1% of children and adolescents now fitting in this category, according to the NIH.
Sadly, the problem will only worsen. A recent study published in the New England Journal of Medicine projected that by 2030, ~50% of US adults will have obesity and nearly half of them will have severe obesity. Other estimates predict 80-90% of the US population will have overweight or obesity.
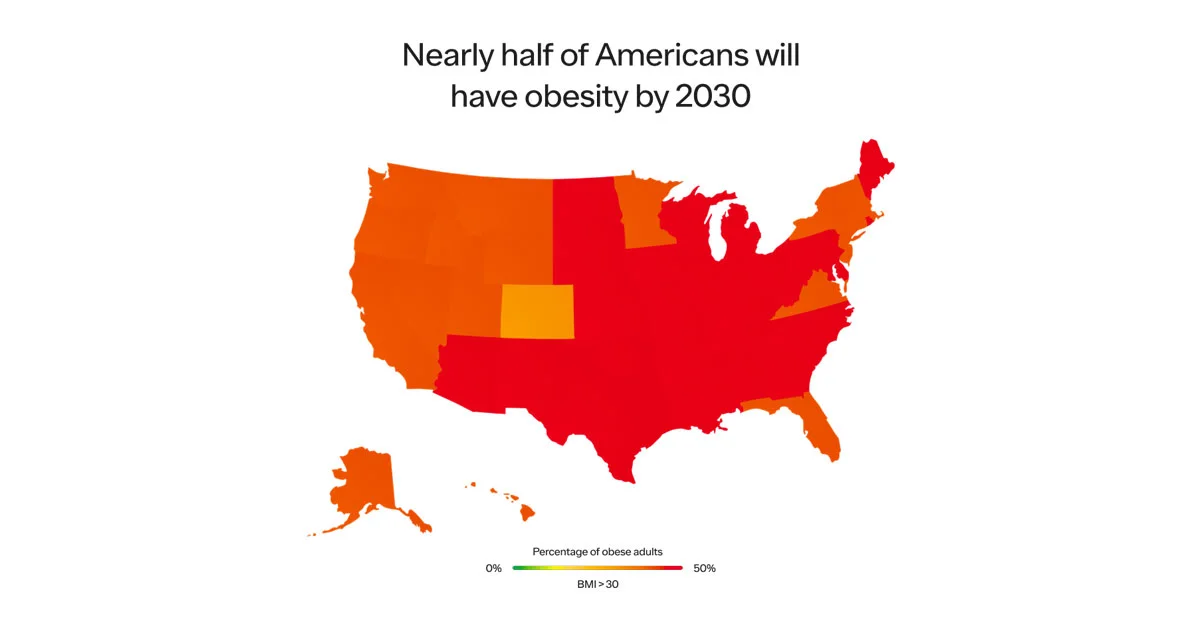
SOURCE: HARVARD SCHOOL OF PUBLIC HEALTH
Cost:
While unnecessary suffering and premature death are top concerns, obesity also has major economic consequences. The CDC estimates direct medical costs of obesity at $173B per year (in 2019 dollars) with added productivity costs to range between $3.38B and $6.38B per year. According to a report by the Milken institute, the total cost of chronic diseases due to obesity and overweight was $1.72 trillion—equivalent to 9.3 percent of the U.S. gross domestic product (GDP). Estimates vary, but the magnitude is significant.
With safe and effective treatments for obesity available, it’s imperative that society help those with this chronic condition live the fullest and longest lives they can while decreasing the burden on society at large.
Why now?
Obesity isn’t a new problem. As mentioned above, obesity has been increasing unabated for the past five decades in both children and adults, and we’ve been paying the price with poor health outcomes and greater healthcare costs.
But it seems to have captured the cultural zeitgeist in the last few months. Why?
For the first time, we have both an effective and potentially scalable solution to help millions of people struggling with obesity.
The key to solving any problem that impacts hundreds of millions of people is to have a solution that is both extremely effective and extremely scalable.
You can visualize these solutions best on a 2x2, with efficacy on the x axis and scalability on the y:
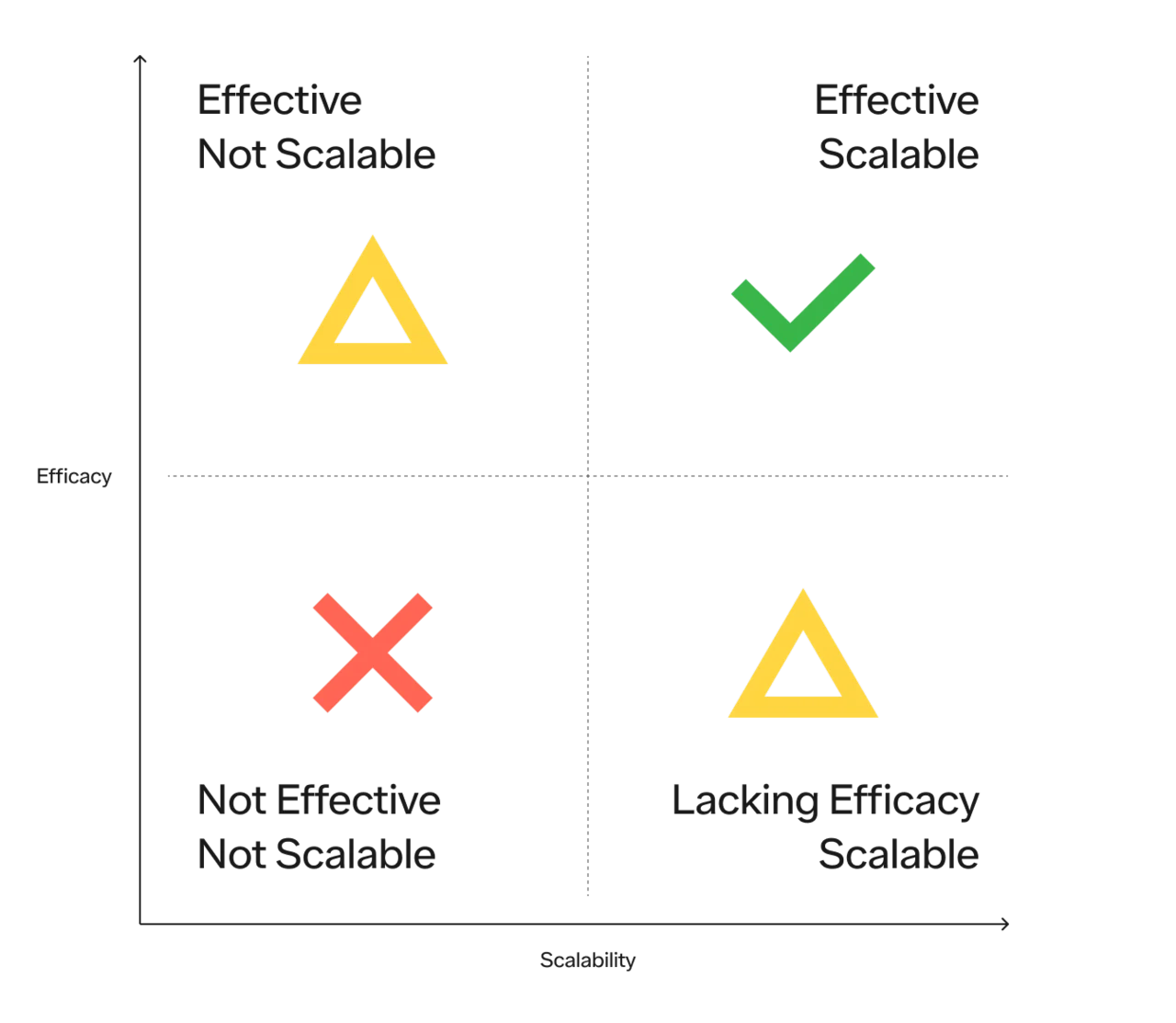
There are 4 quadrants:
Not effective and not scalable (useless)
Effective but not scalable (useful for some, but inaccessible to many)
Scalable but lacking efficacy (progress but insufficient)
Effective and scalable (the holy grail)
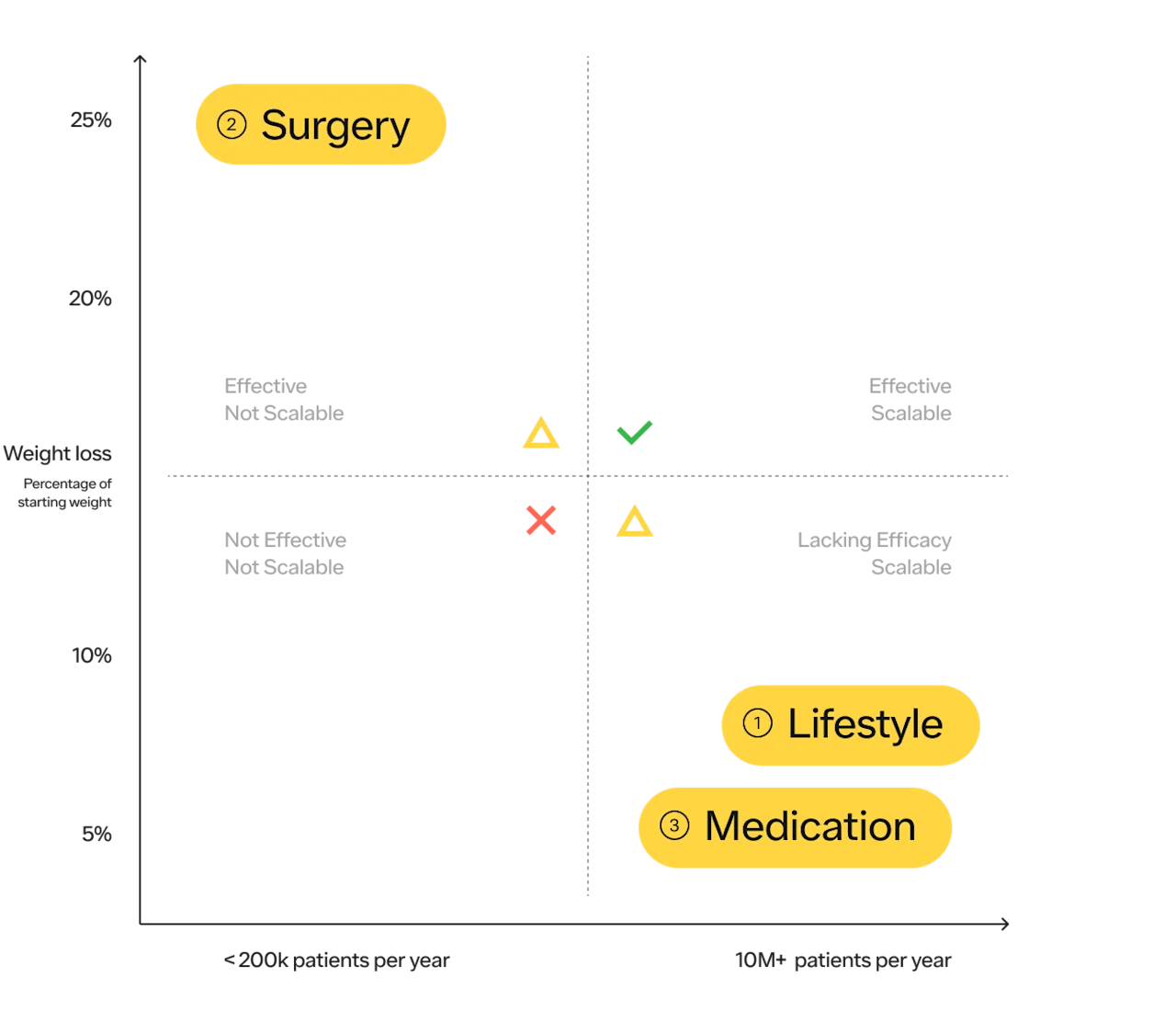
Historically, there have been 3 main approaches to obesity treatment;
Lifestyle modifications consisting of diet and exercise, and cognitive behavioral therapy
Weight loss surgeries (sometimes referred to as bariatric surgery or metabolic surgery).
Medications that promote weight loss
Let’s go through each.
#1 Lifestyle modifications:
Diet and exercise are the most obvious choices for most people and are important to promote health. Make no mistake, they are amazing for your overall health, regardless of their impact on your weight. Everyone should exercise. Unfortunately, in the treatment of obesity, research shows diet, exercise, and behavioral counseling typically cause modest to average weight loss of 5–10% of body weight with up to 80% of the weight regained after 5 years. While it may work for some, at the population level it has not been an effective tool for a variety of reasons. More on the considerations of diet and exercise as it relates to obesity prevention and treatment below.
#2 Surgery:
In terms of efficacy, surgery is number one, with weight loss averaging 20–30% of body weight depending on the type of surgery. Surgery has also shown benefits for long-term health, decreasing the risks of many types of cancer as well as diabetes, heart disease, and all-cause mortality.
The American Society for Metabolic and Bariatric Surgery (ASMBS) and International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO) recommend bariatric surgery for all patients with a BMI of ≥ 35 kg/m2 or those with BMI of 30-34.9 kg/m2 with metabolic disease (2022 Guidelines).
While surgery is the most effective solution, it is the least scalable.
According to the ASMBS, approximately 250k bariatric procedures are performed in the US each year. With ~23M US adults with severe obesity (BMI > 39.9 kg/m2), it would take over 90 years to complete surgeries on this population alone! While we hope everyone who is suitable for a treatment, and wants that treatment, can access it — the practical reality is that it is not the case. Bariatric surgery is clearly not a solution that can be scaled for the population that can benefit from it.
#3 Medications:
Drugs to promote weight loss have been used for at least 100 years — with varying efficacy and safety profiles — and have a complicated history that has subsequently created a negative perception of “weight-loss meds,” some of it well justified.
In the first half of the 20th century, amphetamine and 2,4-Dinitrophenol (DNP) were used for weight loss. Amphetamine had many unwanted side effects and became a schedule II drug in the 1970s due to its addictive potential. DNP was withdrawn from the market due to serious side effects, including death. Later, amphetamine was chemically modified to reduce unwanted side effects while retaining its appetite suppressing properties. These drugs included Aminorex, Phenylpropanolamine, and Fenfluramine. Aminorex was withdrawn from the market in 1972 after it was found to cause pulmonary hypertension (a dangerous increase in the blood pressure in the blood vessels of the lungs), and fenfluramine, part of the infamous drug “fen-phen,” was withdrawn in 1997 after it was found to cause damage to heart valves. Other weight loss drugs eventually withdrawn from the market due to dangerous adverse events include sibutramine (heart attack, stroke), rimonobant (depression, anxiety, suicidal ideation), and lorcaserin (increased risk of cancer).
Currently, there are three amphetamine derivatives still approved for weight loss: diethylpropion, benzphetamine, and phentermine, which is the most popular of the three. All three drugs are approved only for short term use and have limited potential due to their high burden of side effects and contraindications in people with health problems, including those common in people with obesity (e.g., hypertension, heart disease). There are also several medications (e.g., metformin, bupropion, naltrexone, topiramate) that are used alone or in combination, on and off label, that have been shown to lead to 2%–10% weight loss in certain populations.
This long, checkered history of anti-obesity medications led to wariness on the part of consumers and healthcare professionals. So many of these drugs either turned out to be dangerous, have intolerable side effects, or were not very effective. However, in the late 20th century, scientists began to better understand the pathways that regulate appetite and energy regulation, and more effective, safer therapies became available.
Qsymia, a combination of lower dose phentermine (to decrease side effects) and extended release topiramate (a drug used to treat seizures and migraines), was approved in 2012 after it was shown to cause ~10% weight loss in a clinical trial. While generally safe, Qsymia still has unwanted side effects in many patients (e.g., constipation, insomnia, headache) and can cause birth defects if taken by pregnant women. Contrave, a combination of the antidepressant bupropion and naltrexone (used for opioid and alcohol addiction) was approved in 2014. Weight loss from Contrave is modest at ~5% over placebo. In 2019, the FDA cleared Plenity, a weight loss device taken by mouth as capsules. Plenity is taken twice per day with meals and was shown in a clinical trial to cause an average 6.6% weight loss in all users and ~10% weight loss in responders.
As you can see from the brief summary above, there is a recurring pattern of weight loss drugs being approved and then withdrawn. So, the skepticism people have that there is “finally a safe and effective weight loss drug” is not unwarranted based on the history. We’ve thought we had an answer before, so it’s fair to ask, “why is now any different?”
The answer is in our better understanding of the science of the causes of obesity and how new medicines address one of the underlying causes. We also have a track record with the class of GLP-1s, which have been around for 18 years in different forms and have been used to treat diabetes safely. GLP-1s have even been shown to decrease the risks of cardiovascular events, like heart attacks. But they do not come without side effects, including an unknown risk of thyroid cancer in humans and contraindication for patients with a history or family history of a specific type of thyroid cancer, called medullary thyroid carcinoma. The most frequent side effects are GI-related, such as nausea and vomiting, and should resolve over time. More serious side effects like pancreatitis and gallbladder disease can occur, but are rarer. The risk profile of GLP-1s should be weighed appropriately against the benefits and in consultation with your provider (see Wegovy safety information here). With a better understanding of obesity and how these medications treat it and a track record of safety, we are in a different starting place this time around.
Continued close scrutiny is justified and helpful. Side effects that do not appear when many thousands of patients have used a medication may appear when hundreds of thousands have been treated or when treatment has been used for many years. Therefore, it is wise to remain vigilant. But the advent of GLP-1s represents a new frontier in the fight against obesity. We finally have a treatment that is both effective and feasibly scalable (holding cost aside for a moment).
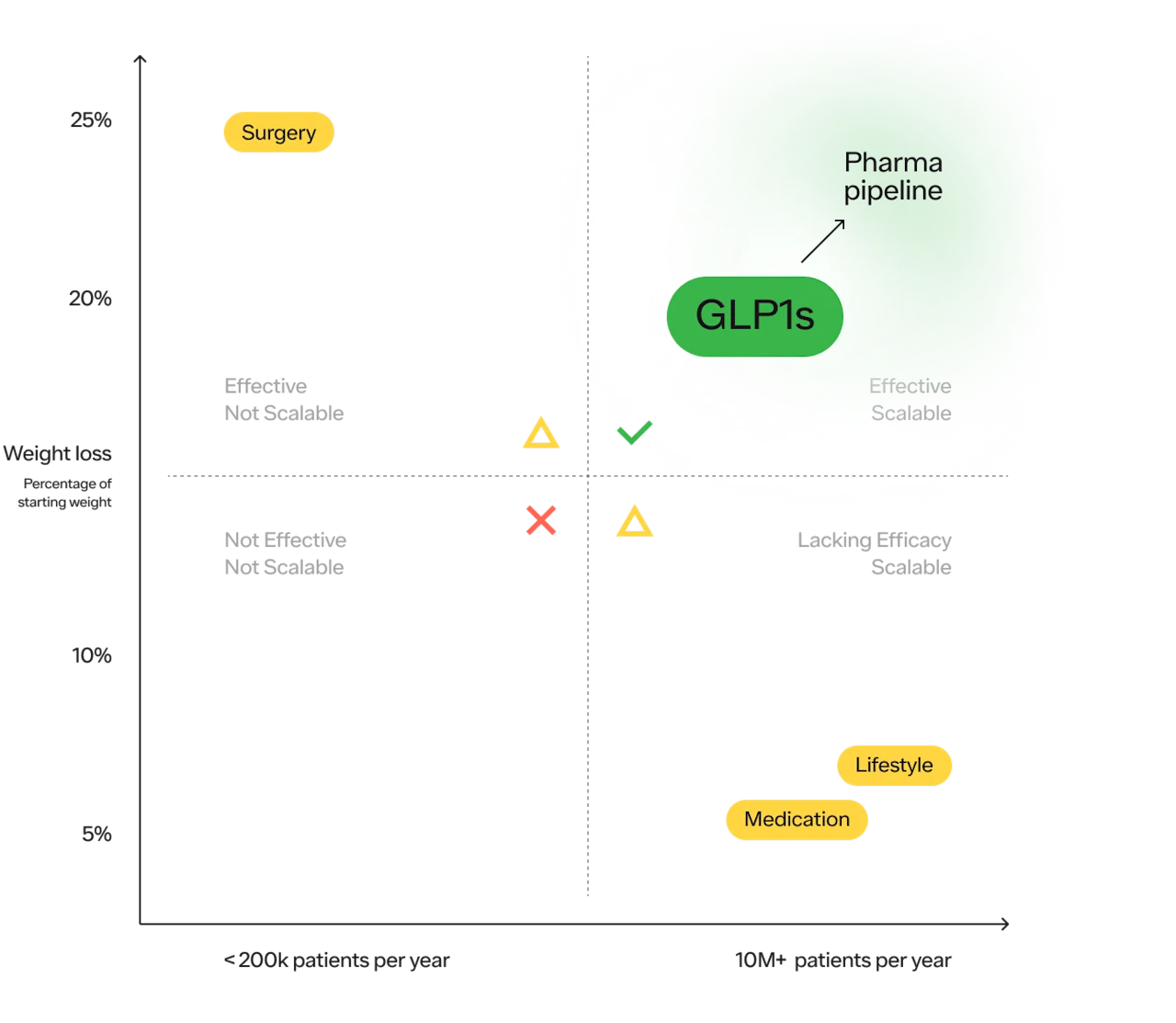
And there are more medications on the way that fit within the upper right quadrant. Between Novo Nordisk and Eli Lilly, the makers of Wegovy (Semaglutide) and Mounjaro (Tirzepatide) respectively, there are 12 more obesity medications in development.
—
GLP-1 drugs were originally developed to treat type 2 diabetes, with exenatide being the first GLP-1 approved for type 2 diabetes in 2005. Later, GLP-1s were found to cause modest weight loss in people who took them for diabetes, and a higher dose of liraglutide was found to cause clinically significant weight loss in people without type 2 diabetes when used in conjunction with diet and exercise. This resulted in liraglutide (brand name Saxenda) being the first GLP-1 approved for weight loss in 2014. Liraglutide has several benefits over older obesity drugs, including its beneficial effects on blood glucose and a better side effect profile. However, since it has a halflife of ~13 hours and is available only as an injection, it must be injected daily, which can be inconvenient for patients.
The science of GLP-1 agonists continued to progress, and semaglutide was approved for type 2 diabetes in 2017. Semaglutide has a halflife of ~7 days, and therefore needs to be injected only once weekly. Semaglutide was later studied for weight loss in a series of studies called the STEP trials and was found to cause an average of 15–18% weight loss in non-diabetic patients with an acceptable safety profile. These effects remained for at least 2 years when people remained on the drug.
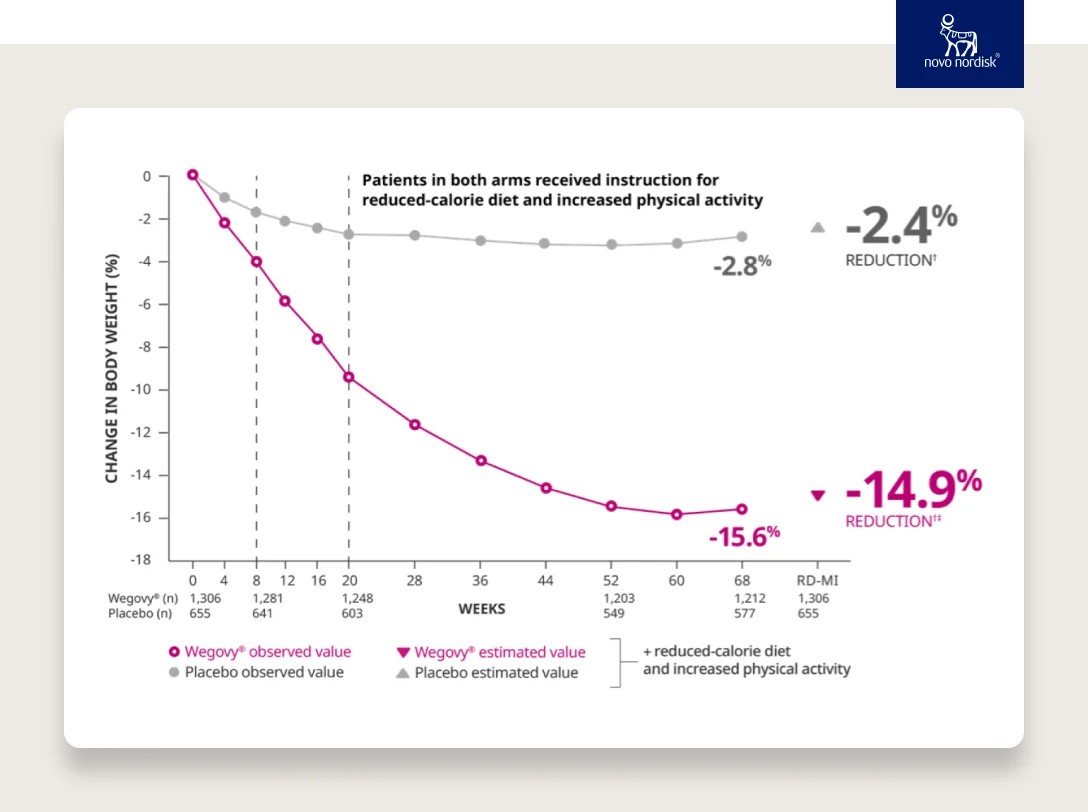
SOURCE
With an average of 15% weight loss, GLP-1s are a game changer. Many patients will lose even more weight.
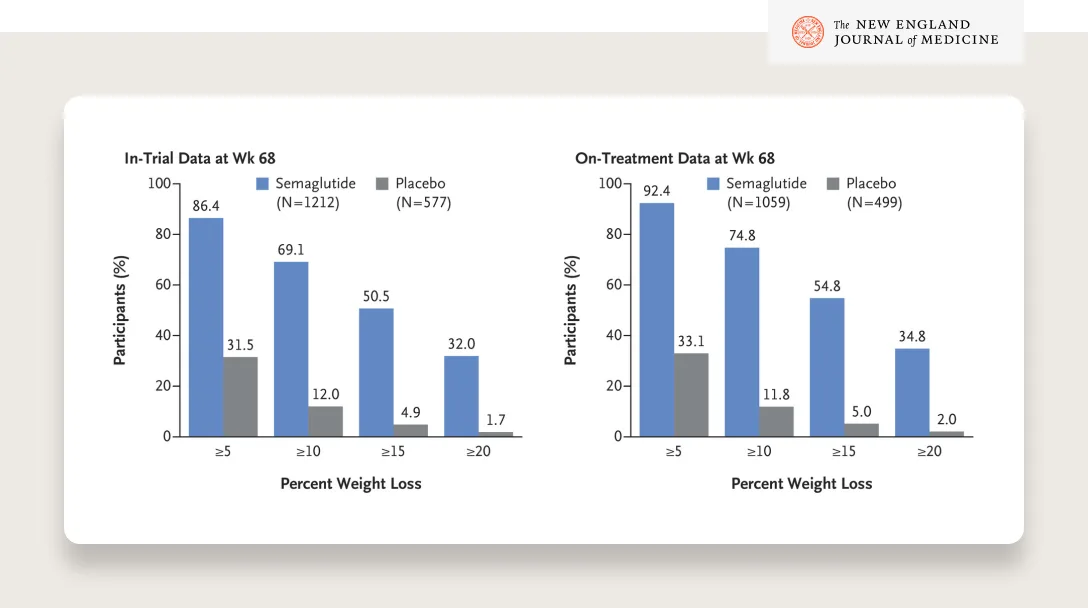
SOURCE
Look at these results — 50% of patients lost more than 15% of their body weight and a third lost more than 20%!
These drugs have the potential to completely change the landscape of the field of weight loss and greatly improve the health of those with overweight and obesity. Research into GLP-1 and the family of hormones it belongs to, called incretins, continues. In the future, there is likely to be a larger selection of effective anti-obesity medicines appropriate for different individuals with different medical histories. Some may even be oral medications.
The STEP trials showed that semaglutide (2.4mg dose) resulted in an average weight loss of 15–18% bodyweight with good tolerability and sustained weight loss for at least 2 years. Tirzepatide (brand name Mounjaro), a dual GLP-1/GIP agonist, has shown > 20% weight loss in a clinical trial and is likely to be approved for weight loss sometime in 2023.
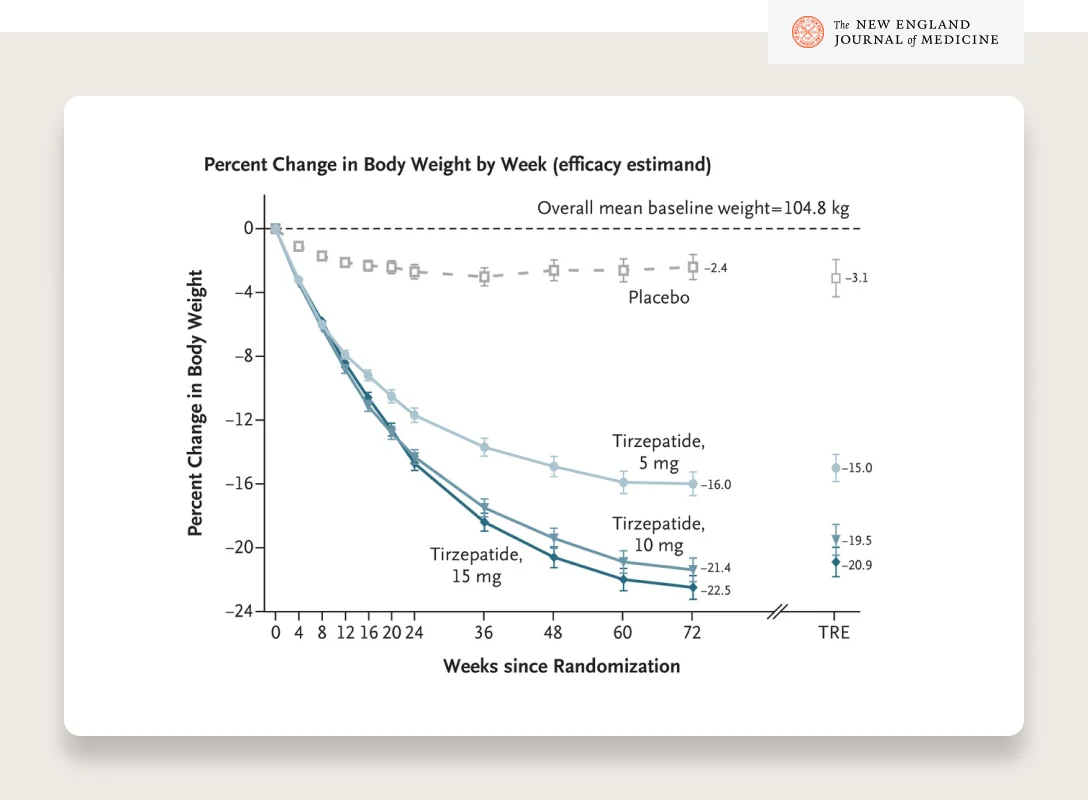
SOURCE
We finally have medications that can be deployed at scale to help the tens of millions of patients affected by obesity. Now is the time to offer patients the treatment they deserve.
In layman’s terms, how do GLP-1s help people lose weight?
Your body naturally makes GLP-1. It’s a natural hormone your gut releases after meals. The hormone acts on the pancreas to increase insulin secretion and decrease glucagon secretion (glucagon stimulates sugar production) in response to food, keeping blood sugar from rising too high. This medication, a “GLP-1 agonist,” strengthens that response when you eat. It helps you lose weight in three different ways:
Helps increase the amount of insulin released after eating (keeping blood sugar low)
Hunger increases on the back side of glucose spikes
Slows the rate food leaves the stomach and heads into the small intestine
If the food stays in your stomach longer, you’ll feel full earlier and longer
Sends a signal to your brain that acts on the reward center of your brain, decreasing appetite and cravings for high-calorie foods
This is considered the primary way GLP-1s help people lose weight and keep it off (more on keeping it off below).
For patients with diabetes, these medications have also been shown to have greater health effects than weight loss by:
Lowering A1C
Lowering blood pressure
Lowering LDL
Raising HDL
Lowering risk of heart attacks by 26% relative to placebo (SUSTAIN 6)
The SELECT trial is currently studying these medications for cardiovascular protection in people with obesity who do not have diabetes. Many are hopeful we’ll see reductions in cardiovascular events in non-diabetic patients as well.
But don’t people regain the weight when they stop taking the medication?
Typically, yes. The data shows that the majority of patients will regain the weight they lose when they discontinue use.
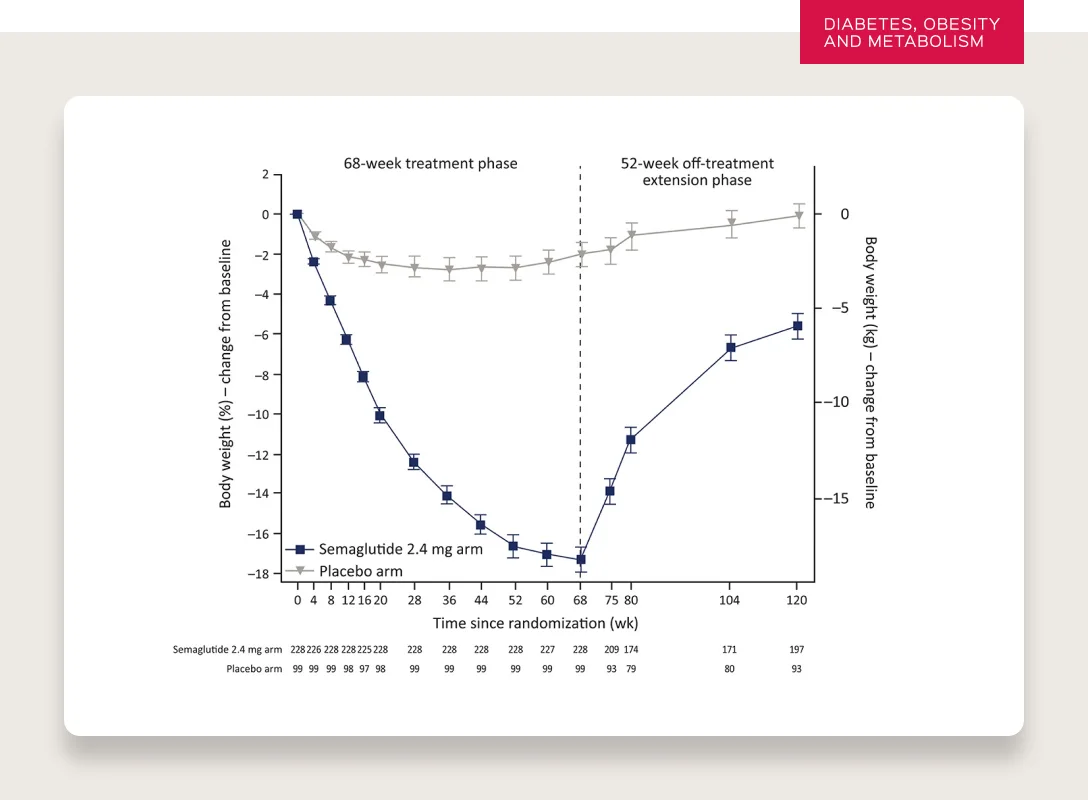
SOURCE
It’s worth understanding why this occurs. But before I explain, I want to note that the question itself reveals a subconscious bias against viewing obesity as a disease. Most medications for chronic conditions require continued use to maintain efficacy — from hypertension to diabetes to lipid-lowering treatment. If a patient stops taking their medication, they will stop seeing the benefits.
The expectation that individuals should be able to maintain their weight loss without treatment again highlights the widespread, but mistaken social view of obesity more as a flaw of willpower and self discipline, rather than a complex combination of genetics, epigenetics, environment, lifestyle, and social determinants of health.
The reason this is so important to understand is that while some patients will be able to go off a GLP-1 and maintain their weight loss (which is amazing), not every patient will and they shouldn’t feel any less adequate as a result.
Of course, we all hope that someday we find ways to cure the disease of obesity and other chronic diseases without the continued use of medication. But the expectation or surprise that this isn’t the case with obesity does highlight that we as a society do not view obesity as we do other diseases.
Now let’s talk about why patients typically see weight regain after discontinuing use:
Losing weight is difficult, very difficult, but it is not the hardest part of weight management. Maintaining weight loss after losing is the hard part. We all know this intuitively. Most of us know many people who have lost weight—sometimes hundreds of pounds—only to regain it. Why does this happen? While some of the details remain obscure, scientists have developed a theory called “weight set point” to explain why maintaining weight loss is so difficult.
Set-point theory explains that our bodies have a specific weight they strive to defend, and if we lose a substantial amount of weight, our bodies work hard to regain weight until we return to our set-point (i.e., the level of body fatness our body “wants” us to have). This happens through a variety of mechanisms. Our hunger hormones increase, making us eat more, while our resting energy levels go down so we burn less energy at rest. We may also have less desire to exercise or move around, also making us burn less energy. Our body mimics a starvation response.
Unfortunately, the reverse does not occur to the same extent. When people gain weight over the course of a lifetime, mechanisms do not kick in to make us lose this excess weight. Scientists have different theories explaining why we defend weight loss more than weight gain, causing humans to be so susceptible to weight gain and obesity.
One theory called the “thrifty gene hypothesis” states that famines caused the selection of genes that make it more likely for us to gain weight so we can survive when food is scarce. These genes are so common in the population because people with these genes were more likely to survive and have children than people with genes that caused them to remain lean. We selected for the genes that allowed us to gain weight, stay that way, and procreate.
Another theory called the “drifty gene hypothesis” (more unique names would be nice 😂 ) states we are genetically susceptible to weight gain because we developed technology (fire, weapons, language) that allowed us to evade predators. This meant that the normal evolutionary pressures that would select the leanest, fittest, and fastest humans to survive were removed. The removal of pressures favoring lean genes allowed genes favoring weight gain to remain in the human population.
We don’t know whether we “selected for body fatness” intentionally for the purposes of survival and procreation or whether we “no longer needed to select for body fatness” so it remained in the gene pool rather than being eliminated through natural selection.
While we don’t know for sure what has caused humans to be so susceptible to obesity, it is clear that evolution is more concerned with survival and reproduction than it is with protecting against the long term consequences of overweight and obesity.
The net result is our bodies fight more to prevent weight loss than weight gain. We don’t put up much of a fight when we gain weight (we even make it really easy), but our bodies fight incredibly hard to make sure we don’t lose weight (whatever weight we settle in at — “the set point”).
This makes sense from an evolutionary standpoint. Humans have spent close to 99.5% of our history as hunter/gatherers, 0.4% of our history as farmers, and .1% of our history in the post-industrial revolution. We have not evolved for our modern food environment with a plentiful supply of cheap, calorie-dense, extremely delicious food not found in nature (scientists call this type of food “hyperpalatable").
Whatever the cause, our genes have caused us to be much more susceptible to weight gain than weight loss and, for the first time in human history, our food environment has provided us the means to gain that weight very easily, quickly, and cheaply. We talk more below about the changes in our environment and how our brain reacts to this hyperpalatable food (it loves it) but we just scratch the surface on both of these topics.
One of the main ways GLP-1s help us lose weight is by resetting the set-point to a lower weight. They do this by making us less hungry so we lose weight until we reach that new set-point. They help us fight the starvation response with which our body would normally react, making it easier for people to eat less and to form new, healthier habits.
This is why even those taking GLP-1s eventually plateau after losing weight for a year. Once we hit the new set-point, we stop losing weight. However, as discussed above, the new set-point only remains so long as we continue taking the medicine. This is why people regain weight after stopping the medicine. While this is true for GLP-1s, it is also true for other methods of weight loss (and other interventions for chronic diseases). Once the intervention stops, most people regain the lost weight. There are very few interventions in life, let alone healthcare, where you can stop them and continue to see the benefits.
The only method known to maintain weight loss after the intervention in most patients is bariatric surgery. The reason is simple. The intervention is ongoing because the anatomic changes caused by the surgery remain. But again, surgery is not for everyone and unfortunately inaccessible for most (~250k done per year).
Are GLP-1s for everyone?
No.
GLP-1s, the drug class, have been approved by the FDA and used by patients for ~18 years. They have been approved for use in several diseases (e.g., diabetes, obesity) and are appropriate for use by certain people under specific conditions. It is only since 2021 that some of them have been approved for the treatment of obesity.
For the treatment of obesity, Wegovy (generic drug name semaglutide) is approved in patients who have a BMI greater than 27 with comorbidities, or a BMI greater than 30. Even for certain patients within that criteria, they may not be appropriate for treatment given their family history or medical history with thyroid cancer, for example (more safety information here).
This medication is not intended for patients who “just want to lose a few” before a special occasion. But it’s important that this does not stain our perception of the benefit of the medication for those who truly need it.
Healthcare providers generally believe that if there is a net benefit to a patient, meaning a benefit/risk ratio that leans heavily toward a net benefit—as determined by the patient and their provider—then a patient should have the opportunity to receive treatment. In addition, patients should also receive guidance, support, and whatever treatment may be ideal to mitigate the downsides in order to maximize the benefit.
Of course, for some people the risks outweigh the benefits. Just because there are significant benefits, does not mean we do not have to weigh potential risks against those benefits for each patient. But the overall calculation (i.e., benefits vs. risk) involves the same process for any medication.
To be clear, there are also people for whom treatment with GLP-1s is beneficial but not sufficient on its own. Obesity is a serious disease that, due to its complexity as well as the body's ability to resist sustained weight loss (more on that later), can require multiple treatments to address. Treatments, whether lifestyle modifications, surgery, or medications, should be seen as tools in a toolbox from which patients and providers can pull from together to build the best possible treatment plan.
What about loss of lean mass from GLP-1s?
Some amount of lean mass loss is inevitable with significant amounts of weight loss. People with obesity tend to have more lean mass, including muscle mass, and fat mass than people without obesity. In the process of weight loss, people will lose both lean and fat mass. However, individuals will generally lose more fat mass than lean mass. Overall, this is a benefit for the person as the composition or ratio of fat tissue to muscle tissue decreases. This occurs with all forms of significant weight loss.
Let’s run through a few examples: bariatric surgery, calorie restriction, and the use of GLP-1s.
Bariatric Surgery
Bariatric surgery often results in a loss of lean body mass, including muscle mass (systematic review and meta analysis) in patients, and yet large studies have shown that bariatric surgery lowers the risk of heart disease, diabetes, and all-cause death with long-term follow up. So, while a loss of lean mass occurred after surgery, the intervention and accompanying weight loss led to an improvement in overall health outcomes.
Calorie restriction
Weight loss due to calorie-restriction has also been shown to produce varying amounts of loss of lean body mass. Notably in this study from 2019, women on a low calorie diet paired with physical activity noted improved body composition and decreased muscle mass loss at 3 months, and even some muscle mass increases in patients with more intense exercise regimen.
GLP-1s
In the “Lean mass loss on GLP-1 receptor agonists: a downside of the ‘miracle drugs’” Peter Attia states, “[t]hough a certain amount of lean loss is inevitable with significant weight reduction (usually about 25% of total weight loss), the goal is to increase the body’s overall proportion of lean mass – in other words, to improve body composition”
While the data so far are limited, the data in the STEP 1 trial for semaglutide show that lean muscle mass accounted for ~39% of the weight loss, which is undoubtedly higher than ideal. Having said that, the proportion of lean mass to body mass still increased (~3% in STEP 1 trial and ~1% in SUSTAIN 8), which means patients are losing weight and their body composition is improving as well (the ultimate goal). It should be noted that the trials done for the use of GLP-1 medications for weight loss also included instructions to pursue an exercise regimen and lower calorie diet along with use of the GLP-1 medication.
In the same study, of the weight loss by the placebo group (page 25, table S5), 57.2% was lean body mass. The placebo group actually saw a greater percentage of lean body mass loss. However, they did lose less overall lean body mass (they also lost less overall weight and fat mass). As a result, it’s difficult to say that GLP1s are the cause of the lean muscle mass loss or that they are protective of it.
Why does this matter?
As noted above, many patients who have obesity have more lean muscle than patients who do not have obesity. Due to this fact, patients with obesity may be able to better withstand the loss of lean muscle mass associated with weight loss by any method.
That said, some patients may be at greater risk than others when losing lean muscle mass. A key example of this are older patients. Patients with obesity aged 65 and above may have less lean muscle mass as a facet of age, injury, or limited mobility due to other health conditions. While a patient in this age group may have a BMI in the obese range, it does not necessarily mean they have the same amount of lean muscle mass as a younger person at the same BMI. This loss of muscle mass, particularly in older persons, has a name: “Sarcopenia.” Decreasing muscle mass can result in decreased muscle function. For an older person, this can be very challenging due to their decreased ability to rebuild lean muscle mass as well as the enhanced risk of additional medical conditions or injuries associated with decreased physical function.
What do you do about it?
While overall health outcomes can be improved with weight loss and the accompanying lean muscle mass, and people with overweight and obesity can often afford to lose additional lean muscle muscle, it’s still very important to try to maintain as much lean mass as possible for maximum metabolic health and prevent frailty in older age.
With regards to GLP-1 use, the loss in lean muscle mass highlights the importance of exercise, particularly resistance training, which may mitigate lean muscle mass loss while using GLP-1s (additional protein consumption is also helpful). It also might influence which patients receive GLP-1s and which specific GLP-1 medication if certain GLP-1s lead to less lean muscle mass loss than others.
It is important to emphasize that the downside of any drug should be factored into the risks and benefits trade off for any patient. For some patients, even if they were unable to participate in resistance training, it wouldn’t mean that they should be restricted from receiving treatment with the medication; it just might mean they would see better outcomes if they did resistance training as well. For others, for example someone with extreme sarcopenic obesity (excess fat with low muscle mass), GLP-1 treatment might not be best for them until they can increase their lean muscle mass. Of course, if you have any medical questions or concerns, please talk to your healthcare provider. The information contained in this article is not a substitute for professional medical advice, diagnosis, or treatment.
Last but not least, studies have shown the best way to decrease the loss of lean body mass when losing weight is a combination of exercise and higher protein intake. Aerobic exercise and resistance exercise both show a muscle preserving effect, but resistance training is better at preserving muscle. The good news is studies show that exercise and moderate to high protein intake can help decrease loss of lean mass even in older people who are at the greatest risk of losing too much muscle when losing weight.
Doesn’t this take medication away from people with type 2 diabetes?
Everyone who needs access to medication should have access to it. Full stop. No one ever wants a patient to lack access to their medication because of a shortage. The idea that this is a competition between people with diabetes and people with obesity, as opposed to a need to supply medication to multiple groups that need it, again shows the widespread bias against people with obesity and our society’s failure to view obesity as a diseaseIf the shortage is caused by people who should not be on the medication, then of course this is problematic. However, I believe it’s more likely that shortages are the result of unanticipated demand by patients with obesity and/or diabetes who have only recently become aware of this revolutionary medication.
According to the CDC’s National Diabetes Statistics Report 2020, 89% of patients with diabetes had overweight or obesity. 9/10 people with diabetes have overweight or obesity.
76% of the U.S. population has overweight or obesity (43% have obesity).
Close to 60% of the US population would fit within the criteria of the Wegovy label
Insurance companies will cover the medication only if they deem it clinically appropriate, and its expense has resulted in their constructing tight guardrails.
It is disappointing that some people who do not clinically need it are using this medication. Even one person doing this during a shortage prevents another person who truly needs it from having access to it. While this does occur, the data suggest the vast majority of the shortage is driven not by people paying $1000/month (again, insurance companies are unlikely to cover those who don’t need it) but rather the sheer volume of people who have diabetes or obesity (or both) and who need and deserve this treatment. As far as “diabetes vs. obesity”:
This is somewhat of a false dichotomy as often they are the same patient (9/10 people with diabetes have overweight or obesity according to the CDC).
50% of all new diabetes cases in the US are linked to obesity (obesity is often upstream of diabetes).
Personally, I’m not in support of debating the moral relativity of complex metabolic diseases (i.e., diabetes and obesity) that seem so intertwined that the biggest differences between the two seems to be how we as a society view the diseases and how we judge the patients who have them.
Again, if you’re asking “diabetes v. obesity” (not about whether those who don’t need it should use it), ask yourself if you’d have the same questions or reactions to the current shortage if this were a treatment for diabetes and heart disease and the patients with heart disease were “causing” the shortage. I don’t think our reaction as a society would be the same. We’d probably ask ourselves — “why can’t we make more?”. The irony is that we have this incredible new treatment that treats the disease associated with 8 of the 10 leading causes of death, and our initial reaction is to admonish patients seeking the treatment. Why haven’t we asked why pharma companies have been unable to keep up with the demand? What does that say about how we view obesity?
What about the cost of these drugs? $1000+ per month is unreachable for most people.
Right now, these drugs are too expensive. That’s absolutely right, and I share this criticism. A few thoughts on the topic:
New technologies take time to democratize
People are criticizing GLP-1s, shaming people who are taking them, and then asking why the medications are so expensive such that not everyone who needs them can get them. With the latter, I fully agree.
It’s unfortunate, but often the most revolutionary technologies are initially very expensive and then, over time, decrease in price. To make matters worse, this process takes longer in healthcare than in almost every other industry. Ideally, we’re all trying to shrink the time between luxury and commodity (but we also shouldn’t be surprised—or discouraged— when something starts as a luxury).
Unless we solve the problem of obesity as a society, the most common forms of obesity will be a thing of the past only for the rich within the next 5 years, furthering inequality in the US healthcare system.
My hope for patients is that two things happen: competition drives down prices, and insurance coverage increases.
Competition driving down price:
Novo Nordisk & Eli Lilly have 12 obesity medications in their pipeline. In addition, Pfizer, Altimmune, Zealand Pharma, Hamni, Regor Therapeutics, Sciwind Biosciences and Viking Therapeutics all have obesity medications in development. Some of these medications are showing early signs of being more efficacious (20%+ weight loss) or more scalable (e.g., oral semaglutide).
Pharma companies are many things, and one immutable thing they are is economically driven. The size of the market will hopefully drive many pharmaceutical companies to enter the space, fight for formulary coverage, or potentially go direct at a lower price.
My hope is that the pharma industry won’t watch silently as two companies create a duopoly for the largest pharmaceutical market to date.
Increased coverage:
Starting in January 2023, federal employees will have obesity medication covered for the first time. But without reductions in price (hopefully through competition), the current price of these drugs will strain the system.
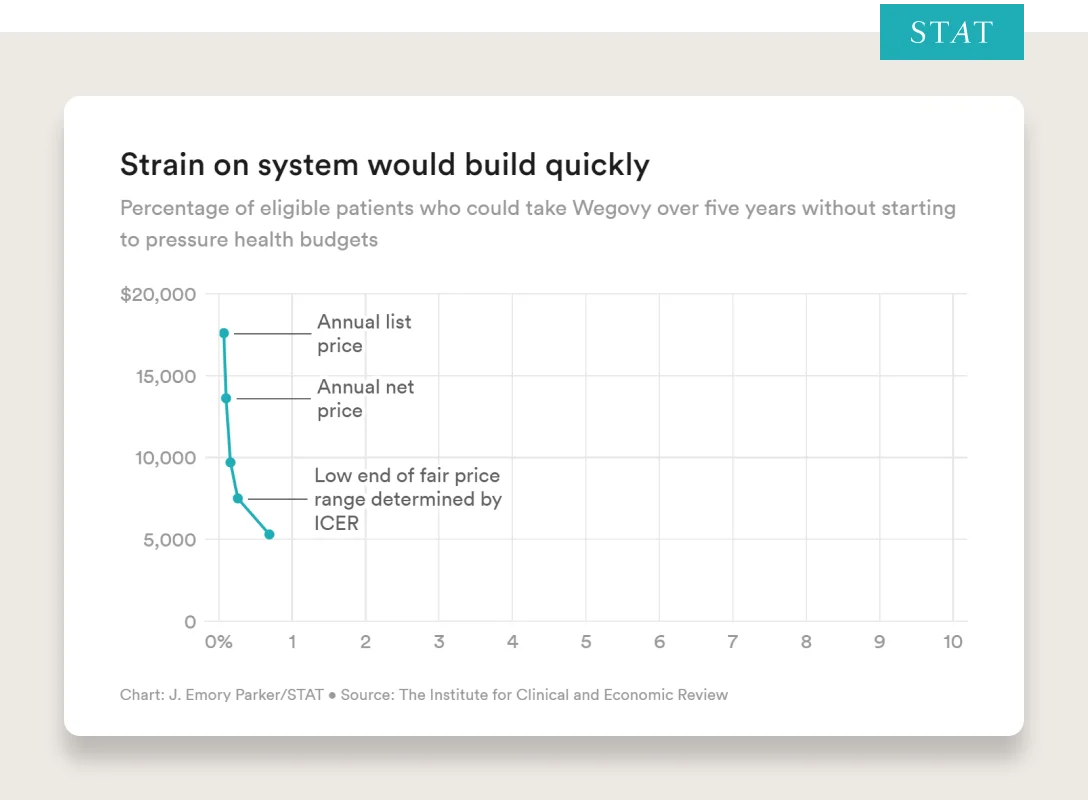
From Stat:
“The Institute for Clinical and Economic Review, a nonprofit organization that evaluates the cost-effectiveness of drugs, issued an alert last fall warning that the U.S. health system is at risk of running up against serious constraints with Wegovy because so many people — almost half the population — are eligible for it.
ICER’s analysis shows that at the drug’s current average net price of around $13,600 a year, as soon as 0.1% of the eligible population takes the drug over five years, insurance companies and government payers may have to shift money or increase premiums.”
Given the long term health benefits and predicted long term reductions in cost, I’m hopeful we’ll see:
Competition reduce price
Medicare and Medicaid follow the government’s lead
Self-insured employers cover treatment and reap long term health, productivity, and cost benefits (opportunity to use as a competitive advantage similar to fertility benefits)
Commercial insurers add coverage to their plans (will be challenging without price reduction for cost not to be passed back to employees)
Aren’t there a ton of unknowns with long term use of GLP-1s?
We can never rule out long term side effects of any drug until we see the data. We can never say with absolute certainty that X will or won’t happen in 50 years with continued use of medication until we study the drug over 50 years. We can make educated estimates, but we cannot know with absolute certainty.
Fortunately, with GLP-1s, we do have safety data stretching back 18 years. Presently, we don’t have any alarming signals of harm that would widely restrict its use, and we have extremely strong evidence of the harm caused by not treating obesity. Part of this evidence comes from studies called “Cardiovascular Outcome Trials.” The FDA requires that drug companies perform the trials specifically to investigate the cardiovascular safety of new medications. Since 2008, new medications authorized to treat diabetes have to undergo these trials which involve thousands of patients and last for years (source). GLP-1 medications are no exception. The results of these trials did not note any increase in cardiovascular events. In fact, there was some suggestion that GLP-1s could improve cardiovascular outcomes in a particularly vulnerable population: patients with Type 2 Diabetes Mellitus. While people are rightfully concerned about the possibility of downstream side effects, this must be balanced by the possibility that there are significant benefits of which we are not aware. This possibility is not idle speculation. For example, recently, medications called Sodium Glucose Transport Protein 2 inhibitors (SGLT2 inhibitors or SGLT2s for short; they are unrelated to GLP-1s) have been approved for treatment of heart failure. This approval was an unexpected result of Cardiovascular Outcome Trials noted above. Though these trials were initially instituted to test the longer term safety of diabetes medications, what researchers found was a significant decrease in hospitalizations for heart failure.
There are many confounding variables when studying obesity. However, and people in the scientific community have come to conclusions of varying strength here, many experts believe the evidence to be overwhelmingly supportive of the conclusion that the amount of body fat or “adiposity” has a major impact on our health based on strong interventional studies (removing the confounding variables most critics use to reduce the impact of body fatness on health outcomes). For example, we see bariatric surgery (holding many factors constant) result in stunning health outcomes — reducing the risk of diabetes by 80% and cardiovascular events by 50% in certain populations.
If we have an effective and safe (based on currently available data) treatment for obesity, with the alternative of doing nothing having known dangers and risks, it again becomes a risk-benefit equation that suggests this solution can be beneficial for millions of people.
Again, we don’t know what happens with continued use for 50 years. But after 18 years, we have solid safety data, and we have strong evidence of what happens if we do nothing. Again, for every individual, risks and benefits should be weighed based on their personal situation with their healthcare provider.
I’ve heard GLP-1s can cause cancer, is this true?
The reason people ask this question, and it’s an extremely appropriate question to ask, is because the following two bullets (in addition to other safety risks) are currently included on Wegovy safety’s label, summarized below:
In studies with rodents, Wegovy® and medicines that work like Wegovy® caused thyroid tumors, including thyroid cancer.
It is not known if Wegovy® will cause thyroid tumors or a type of thyroid cancer called medullary thyroid carcinoma (MTC) in people.
Meaning, in studies of Wegovy on rats, they saw that it caused thyroid tumors but whether or not it will cause cancer in humans is unknown at this point. Having said that, the link in rodent studies between GLP1s and MTC prompted the FDA to mandate continuing studies. In addition, this is why Wegovy is not recommended for certain patients based on their family history “if you or any of your family have ever had a type of thyroid cancer called medullary thyroid carcinoma (MTC) or if you have an endocrine system condition called Multiple Endocrine Neoplasia syndrome type 2 (MEN 2)”.
The risk in humans is still an area of investigation. A recent observational study using data from the French national health system revealed a possible increase of thyroid cancer in patients who used GLP-1 medications. Here are several areas that must be considered in interpreting these results — are GLP-1s the cause? Is this unique to the population of this study or the methods used to conduct the study? Of note, a meta analysis of randomized controlled trial data did not find an increased risk of thyroid cancer among the studies it reviewed. It is clear that our understanding is still evolving.
Another concern is the possibility of an increased risk of pancreatic cancer. Since GLP-1 medications influence the actions of the pancreas, there has been concern that this could cause pancreatic cancer. Reviews and analyses of clinical trials did not reveal a significant increase in pancreatic cancer among patients on a GLP-1 medication.
What about obesity’s link to cancer?
There are strong links between obesity and cancer. “An International Agency for Research on Cancer (IARC) Working Group (NEJM link) concluded that there is consistent evidence that higher amounts of body fat are associated with an increased risk of a number of cancers.” — Cancer.gov. They put together the table below.
Cancer type (reference) | Compared with people without obesity or overweight, this cancer is |
Endometrial | 7 times as likely in people with severe obesity* 2–4 times as likely in people with obesity or overweight |
Esophageal adenocarcinoma | 4.8 times as likely in people with severe obesity 2.4–2.7 times as likely in people with obesity 1.5 times as likely in people with overweight |
Gastric cardia (12) | 2 times as likely in people with obesity |
Liver | 2 times as likely in people with obesity or overweight |
Kidney | 2 times as likely in people with obesity or overweight |
Multiple myeloma | 1.1–1.2 times as likely in people with obesity or overweight |
Meningioma | 1.5 times as likely in people with obesity 1.2 times as likely in people with overweight |
Pancreatic | 1.5 times as likely in people with obesity or overweight |
Colorectal | 1.3 times as likely in people with obesity |
Gallbladder | 1.6 times as likely in people with obesity 1.2 times as likely in people with overweight |
Breast Postmenopausal | 1.2–1.4 times as likely in people with obesity or overweight 1.2 times as likely for every 5-unit increase in BMI |
Breast Premenopausal** | 0.8 times as likely in people with obesity or overweight |
Ovarian*** (26, 27) | 1.1 times as likely for every 5-unit increase in BMI |
Thyroid (28) | 1.3 times as likely in people with obesity 1.26 times as likely in people with overweight |
*RISK FOR TYPE I ENDOMETRIAL CANCER.
**HORMONE RECEPTOR–POSITIVE PREMENOPAUSAL BREAST CANCER.
***HIGHER BMI IS ASSOCIATED WITH A SLIGHT INCREASE IN THE RISK OF OVARIAN CANCER OVERALL, PARTICULARLY IN WOMEN WHO HAVE NEVER USED MENOPAUSAL HORMONE THERAPY (26). THE ASSOCIATION DIFFERS BY OVARIAN CANCER SUBTYPES, WITH STRONGEST RISK INCREASES OBSERVED FOR RARE, NON-SEROUS SUBTYPES (27).
How many of these cancers are due to obesity?
From the National Cancer Institute: “A nationwide cross-sectional study using BMI and cancer incidence data from the US Cancer Statistics database estimated that each year in 2011 to 2015 among people ages 30 and older, about 37,670 new cancer cases in men (4.7%) and 74,690 new cancer cases in women (9.6%) were due to excess body weight (overweight, obesity, or severe obesity) . The percentage of cases attributed to excess body weight varied widely across cancer types and was as high as 51% for liver or gallbladder cancer and 49.2% for endometrial cancer in women and 48.8% for liver or gallbladder cancer and 30.6% for esophageal adenocarcinoma in men.
Globally, a 2019 study found that in 2012, excess body weight accounted for approximately 3.9% of all cancers (544,300 cases), with the burden of these cancer cases higher for women (368,500 cases) than for men (175,800 cases). The proportion of cancers due to excess body weight varied from less than 1% in low-income countries to 7% or 8% in some high-income Western countries and in Middle Eastern and Northern African countries.” — Cancer.gov
It is worth noting the following disclaimer that is, and should be, attached to data linking obesity and cancer. While the links are extremely strong, “Nearly all of the evidence linking obesity to cancer risk comes from large cohort studies, a type of observational study. However, data from observational studies cannot definitively establish that obesity causes cancer. That is because people with obesity or overweight may differ from people without these conditions in ways other than their body fat, and it is possible that these other differences—rather than their body fat—explain their increased cancer risk.” — Cancer.gov
What this means is that there is an association between obesity and cancer, but it does not, for certain, mean that obesity causes cancer. There may be other factors associated with obesity that are the connection between persons who struggle with obesity and those who later develop cancer. That said, it is interesting to note that bariatric surgery has been associated with lower risks of cancer after the surgery. This fact gives some support to the idea that something about obesity is related to a patient’s risk of cancer and that treating obesity can lower it. Many questions remain, such as how much weight loss is necessary to decrease the risk of cancer, how long that weight loss has to be maintained, and is it the same for all cancers. Additionally, we know that persons who lose weight, including those who lose weight with bariatric surgery, may adopt other healthy habits that help decrease the chance of developing cancer. For example, almost all patients who undergo bariatric surgery will receive nutrition education, which includes information about the need to avoid processed food. Use of processed food has been associated with increased risk of breast and colorectal cancer in certain populations. So while weight loss through any means appears to decrease the chance of developing cancer, we can’t say for certain that the weight loss alone will protect someone from cancer.
What about GLP-1 usage in kids? Wegovy was recently approved for kids 12 and up.
This is definitely one of the most challenging topics. Note: Ro only treats patients 18 and up.
In December 2022, the FDA approved Wegovy for adolescents aged 12 and up with an initial BMI at or above the 95th percentile for age and sex (based on CDC growth charts).
Here is what the medical community currently knows — having obesity in adolescence does portend worse health outcomes as an adult. It’s clear that the 12-year-old who might be put on the drug will have bad health outcomes if nothing is done. But it’s unclear what happens when a 12-year-old is placed on Wegovy for 50 years. So again, it comes back to risks and benefits for each patient.
The results of the clinical study of Wegovy for the treatment of adolescents were impressive and received a standing ovation when presented (see the results of the study in NEJM here), but it did also have its limitations. It had only 201 adolescents, compared to the 1,961 adults in the Wegovy study (link to NEJM here).
Regardless of your personal opinion, whether you think the decision to recommend treatment in adolescents was premature or justified, the right decision is not obvious, and two rational experts with the same goal could come to different conclusions. We need look no further than the controversy the new AAP Clinical Practice Guidelines stirred upon release (New York Times coverage). These are extremely hard decisions, and there is a clear need to help these kids, given the likely health challenges they will face later in life.
Of course, there are other methods to help these kids as well. Overall, the obesity discourse would benefit strongly by less often pitting various methods against each other (e.g., lifestyle vs. medication, fix the food supply vs. bariatric surgery) and more often embracing “and” in our efforts (“diet and exercise and medication and fixing the food supply and… and…). There is no reason why all the approaches known to benefit health outcomes cannot be instituted as part of a comprehensive public health effort.
What about “Ozempic Face”?
“Ozempic face” refers to the change in appearance to someone’s face when they lose weight. It’s not specific to medication use and any method of weight loss could have the same result.
In 1895, the Literary Digest warned about “Bicycle Face,” especially for women. They wrote, “Over-exertion, the upright position on the wheel, and the unconscious effort to maintain one's balance tend to produce a wearied and exhausted 'bicycle face”...usually flushed, but sometimes pale, often with lips more or less drawn, and the beginning of dark shadows under the eyes, and always with an expression of weariness.”
Bicycles, especially in the 1890s, were a powerful tool and considered an instrument of empowered feminism. They provided women with a form of agency, mobility, and were so popular that they reduced Victorian restrictions on everyday clothing enabling women to engage in more physical activity with greater ease and frequency. You can read more about this absurd notion in the awesome Vox article here.
I raise this because much of the reporting about “Ozempic Face” sounds a lot like Bicycle face. A scare tactic used by some to stoke fear in a group of people, often a marginalized group, to control or influence their behavior, at least used as a ploy to attract readers. It is typical, even expected, to have excess skin with weight loss. As it is typical to look exhausted after riding a bike. There are many reasons not to do either (GLP-1s aren’t for everyone and I wouldn’t take a bicycle on the autobahn).
From the Vox article — As Munsey’s Magazine put it 1896: "To men, the bicycle in the beginning was merely a new toy, another machine added to the long list of devices they knew in their work and play. To women, it was a steed upon which they rode into a new world."
For many, GLP-1s will be just another drug from pharma. To those suffering and in appropriate need of it, it will be the tool through which they finally know success and can achieve their health goals.
NOTE: Ozempic is FDA-approved to treat type 2 diabetes. It is not FDA-approved to treat weight loss. A provider may prescribe Ozempic for weight loss, if they deem it appropriate to do so in their medical judgment. Ozempic may have serious side effects, including possible thyroid tumors. Do not use it if you or your family have a history of a type of thyroid cancer called MTC or MEN 2. Read more about serious warnings and safety info.
Our genetics haven’t changed in the last 50 years but obesity rates have skyrocketed. How can we say genetics play such a large role in obesity?
George Bray, an obesity researcher, said it well. “Genes load the gun, and the environment pulls the trigger.”
Obesity, like many chronic diseases, has genetic components, environmental components, and our behavior is the confluence. A bit overly reductionist but your genes and environment will influence your subconscious behavior and it’s hard to fully tease these forces apart. There have been books written on this topic; two of my favorites are The Hungry Brain by Dr. Stephan Guyenet and Burn by Dr. Herman Pontzer.
Below, I’ll do my best to simplify, at the highest level, what we understand about the role our genes and environment play and how they have influenced our behavior and ultimately our rates of obesity. Any summary like this will by definition miss certain details so I ask that you bear with me in this broad stroke summary.
My goal is to build an understanding that obesity is a complex interaction between genes and environment, more influenced by things out of our control than you may think. Hopefully, we can build additional empathy for those who have overweight and obesity and add to your understanding of the challenges facing providers, public health officials, and anyone else trying to prevent and treat obesity in society (an important distinction we’ll get to in a later question).
Genetics
“Life is a game of turning energy into kids” — Herman Pontzer.
From an evolutionary standpoint, we’re driven to survive and procreate. To do both, we need energy. “In the economics of life, calories are the currency…and evolution is a heartless accountant” — Burn.
As mentioned above, humans have spent close to 99.5% of our history as hunter/gatherers, 0.4% of our history as farmers, and .1% of our history post-industrial revolution. During that 99.5% of our existence, we didn’t experience an energy-abundant environment. Calories were hard to come by. Obesity wasn’t completely absent, but it was extremely rare.
Whether it was through the thrifty gene hypothesis or the drifty gene hypothesis outlined earlier, or another hypothesis soon to be coined, the grifty gene hypothesis, our genetics are such that we protect far more against weight loss than weight gain. We are much more susceptible to gaining weight in an energy-abundant environment (which has historically been extremely rare), and when our environment changed (described below), we started to see a massive increase in obesity rates.
So, people are correct when they assume “something must have changed,” but incorrect to assume that just because our genetics haven’t changed in 50 years (excluding the role of epigenetics), genetics cannot be partially responsible for the increase in obesity rates. But how much of a role do our genes play? How have scientists gone about estimating the role genetics play in obesity?
—
Like many other diseases, obesity is caused by the complex interaction between our genes, our behavior, and the environment. Each factor affects the other. For example, our genes may affect the likelihood we will behave a certain way in a given environment. When it comes to obesity, one person’s genetics may make them more susceptible to eating a higher calorie diet in an environment where lots of delicious food is always available while another person’s genes may protect them from overeating in the same environment. However, in an environment where only natural, relatively unprocessed foods are available, both people may eat similarly. This shows us how genes, environment, and behavior all interact with each other.
There are also people for whom it seems “no matter how much they eat, they can’t gain weight.” Their body is better at protecting against weight gain. Some of these people, after consuming what their body defines as excess calories, may engage in what’s referred to as NEAT (non-exercise activity thermogenesis), which is the “portion of daily energy expenditure resulting from spontaneous physical activity that is not specially the result of voluntary exercise.” This is a fancy way of describing consistent fidgeting (e.g., leg twitching, changing positions constantly) that can result in a surprisingly significant burning of calories (sometimes 500–800 additional calories). Basically, the body fidgets burn calories and mitigate weight gain when excess calories are consumed.
Studies suggest that BMI (i.e., Body Mass Index calculated by weight in kilograms divided by height in meters squared) is highly determined by genetics, with as much as 60–80% of differences between people being heritable. Yes, almost 60-80% of obesity rates could be the result of genetic susceptibility! How do scientists arrive at that estimate? They study identical twins. Cool, huh? Studies going back decades show that BMIs of monozygotic (identical) twins are much more similar to each other than dizygotic (non-identical) twins even when they are raised apart. This is a powerful demonstration of the strong role our genes play in determining our weight and susceptibility to obesity in the modern food environment.
One more way to think about this is that your genetics play a massive role on where within the BMI distribution curve you’re most likely to fall (i.e., your susceptibility to having a certain BMI compared to the rest of the population).
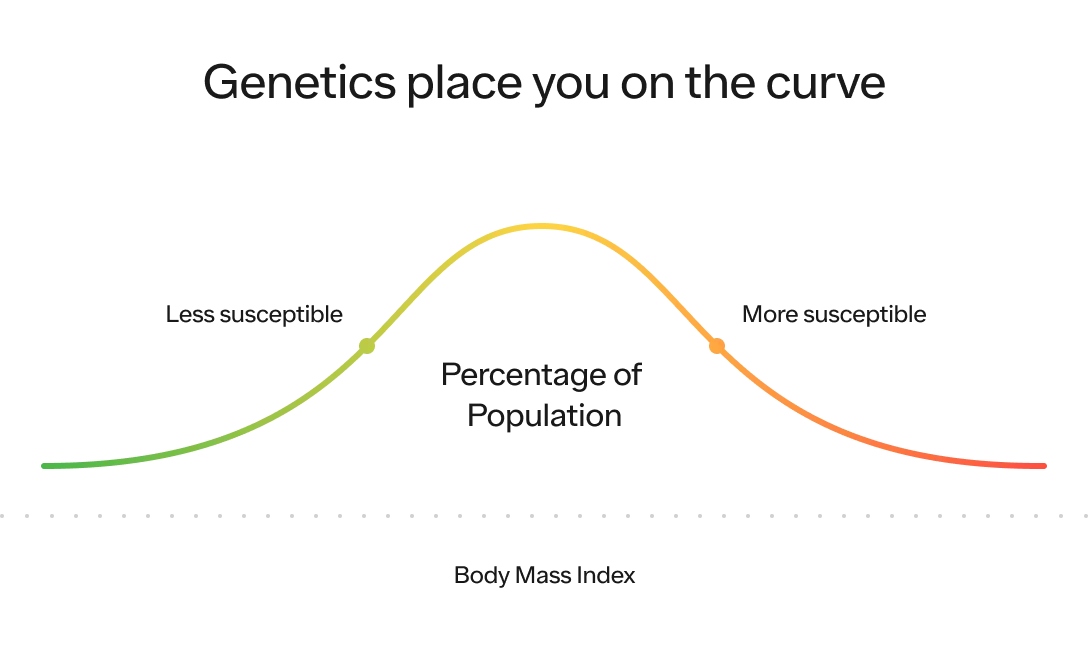
Our environment, which we will dive into below, plays a massive role on where the distribution of the entire population is likely to fall on this curve and how spread out it is. The more energy abundant the environment, the higher the average BMI (i.e., the distribution shifts to the right) and the greater likelihood people will reach the extreme end of the spectrum (i.e., the distribution itself stretches out as well).
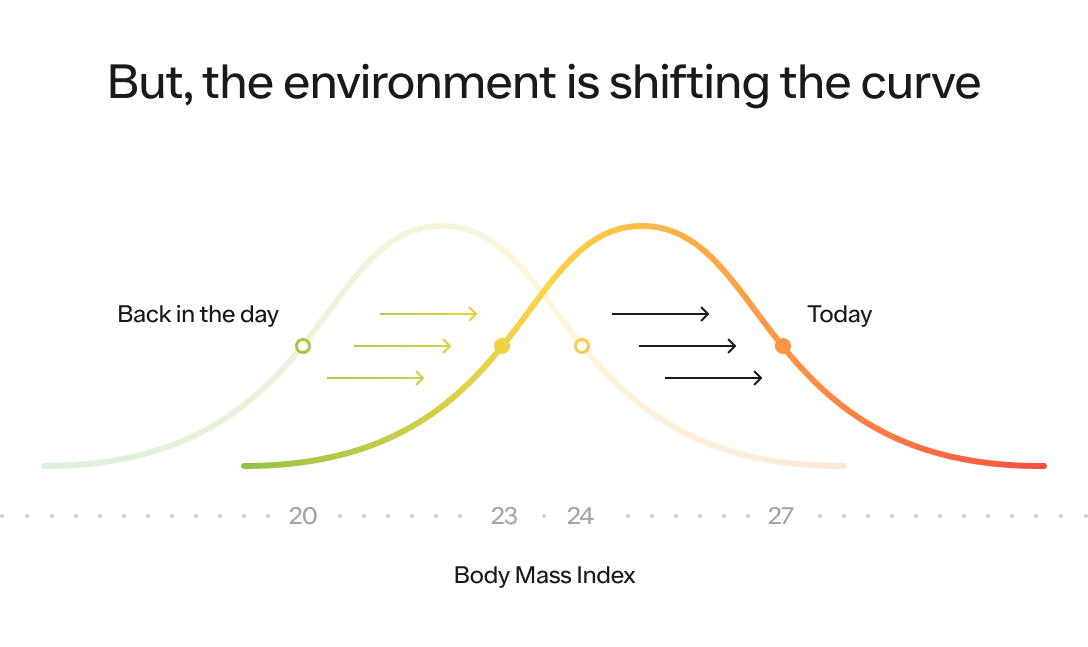
Now, let’s talk about what has changed about our environment that has led the BMI distribution curve of the entire population to shift to the right.
Environmental
Ultimately, a person is able to maintain their weight if the calories (i.e., energy in) absorbed is equal to the number of calories burned (i.e., energy out). The Hungry Brain puts this well:
Energy Balance Equation:
Change in body energy = energy in - energy out
And put another way…
Long term changes in fat tissue = food calories in (and absorbed) - calories out
Calories in
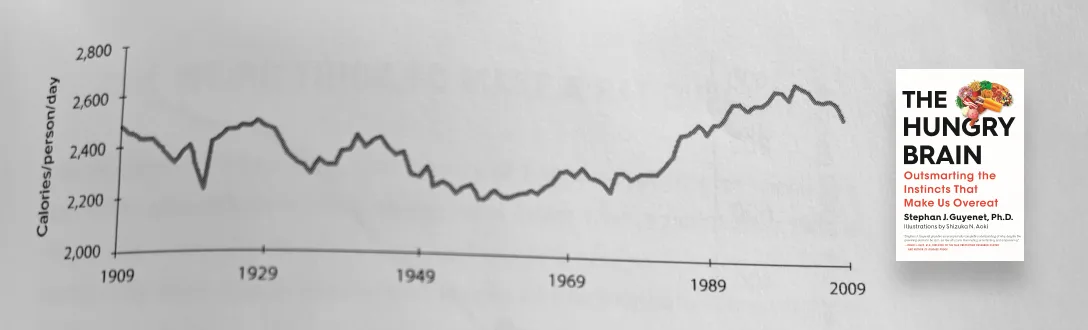
Measuring caloric intake is itself a challenging task. For example, the USDA, the CDC’s NHANES study, and the Hall method, all show a different average calorie intake from 1975 to 2005. But, based on high-level estimates (based on the USDA Economic Research Service estimates), here is a graph showing estimated Calories per person per day from 1909 to 2009.
SOURCE: THE HUNGRY BRAIN
Fascinating! We ate more calories in 1909 than in 1969. But, there was no obesity epidemic in 1909. Why? Let’s look at the other side of the equation — calories out.
Calories out
But what about the amount of energy out? Dr. Stephan Guyenet describes it so well, “In 1890 the United States was a fundamentally different place from what it is today. Farmers made up 43% of the workforce, and more than 70% percent of jobs involved manual labor. Refrigerators, supermarkets, gas and electric stoves, washing machines, escalators, and televisions didn’t exist, and motor vehicle ownership was reserved for engineers and wealthy eccentrics. Obtaining and preparing food demanded effort, and life itself was exercise.”
As the sedentary nature of our lifestyle increased (1909 to 1969), our calorie intake decreased. Life was less exercise and we required fewer calories. We had less of an appetite, we ate less. But notice what happened in the late 1970s. We started to see a rapid increase in calorie intake but our calories out did not keep pace. It was a perfect storm — calorie consumption increasing rapidly while calorie expenditure decreased simultaneously.
When we say “the environment,” again, there is so much complexity here — from our sedentary lifestyle (e.g., Uber, DoorDash, Instacart, Amazon), to Xenoestrogens (e.g., water supply, cosmetics, lithium), to the food supply. They all play a role in our decrease in energy expenditure and increase in energy consumption. They have all contributed to creating an environment favorable to obesity (i.e., obesogenic)
Based on our current understanding, one likely plays a role more than others, and that is the food supply. We have designed hyper-palatable, calorie-dense foods that our brains crave (more on the brain below).
The analogy used in the Hungry Brain describes our doing the equivalent of turning the coca leaf to cocaine and then to crack cocaine but for food consumption — continuously extracting the core ingredient that activates our brain. We’ve created combinations of foods that never existed in nature that are the perfect combinations of sugar, fat, and salt (this is known as “the bliss point”). 5,000 years ago, we’d find calorie dense nuts and our brains would reward us for finding and eating them. We’d also find sweet fruit and know it was ripe. But fat + sweet things did not exist. Now, we can press three buttons and have maple syrup-wrapped bacon delivered to our door.
Food is also much cheaper today. According to data from USDA Economic Research Service, in 1925, the average person spent 23% of their disposable income on food. In 2021, that number was 10%.
Quick refresh:
We’re moving our bodies less
Food has been engineered to press the right buttons in our brain
It’s easier to get
It’s cheaper
We’re reminded of the above constantly
It should be no surprise that nearly 80% of the population will soon have overweight or obesity. The environment is the equivalent of a healthy weight obstacle course.
In addition to our food environment changing, and this topic warrants a much longer discussion but it is worth noting here — “data from the National Health and Nutrition Examination Survey (2017–2018) showed that 20% of US adults are on weight inducing medications”, making maintaining a healthy weight even more challenging.
But before we jump to the brain, it’s also important to acknowledge obesity is the product of human progress. We’ve created this problem by solving others (from infectious disease to transportation to famine). It’s not all bad. This is one of the externalities of solving for scarcity. We’ve created this problem by solving others, and I have no doubt we’ll be able to solve this one together as well. We’ll keep moving up Maslow’s hierarchy of needs together!
Behavior & The Brain
Zooming in even further. How is our behavior influenced by the combination of our genes and our environment? The answer lies within the brain. Obesity is primarily driven by our brain’s response to our environment.
How? At the highest level, it has to do with our brain’s reward-based system and body fat regulation system.
Reward-based system:
Things defined as “good” are reinforced (with a dopamine spike). The first time they occur, we receive a reward after the event occurred, which creates a motivational response that makes us more likely to seek out these things in the future (and vice versa for bad things). Over time, the dopamine transitions to the anticipation of the event occurring as your brain has “learned” this is a “good thing.”
When we eat calorie-dense food (i.e., food with high energy content), our brain sees this as a good thing, we have a dopamine spike, and we have increased motivation for calorie-dense food. This is a healthy response. Calorie-dense food is what allowed our ancestors to survive and procreate.
Body fat regulation system:
In addition to a reward-based system, the brain also has a body fat regulation system. Your body has an ideal percentage body fat in mind (i.e., the set point), and it tries to defend this set point. The main way it defends the set point is by increasing and decreasing your appetite.
How does it do this? Our body has a body fat regulation system called the “lipostat,” and it regulates our body fatness with the primary lever being your appetite.
The lipostat responds to a hormone called leptin that is secreted by fat tissue in your fat cells. The amount of leptin secreted is in proportion to its mass. The more fat in your fat cells, the more leptin they secrete.
Your hypothalamus in your brain has an amount of fat that it wants you to have (i.e., the set point). If you have less fat than it wants you to have, it will increase your appetite and vice versa.
Without going into too much detail, there are 2 groups of neurons in your hypothalamus that control appetite: (1) a group that decreases appetite and increases energy expenditure (leading to weight loss), and (2) a group that increases appetite and decreases energy expenditure (leading to weight gain). Depending on which group is activated (or activated more strongly) will lead you to lose or gain weight (think of it like two sides of a scale). How do we add weight to one side of the scale? We have many hormones released from tissues outside the brain that converge on these 2 groups of neurons that collectively act to determine if appetite is suppressed or increased. Some of these hormones include leptin, GLP-1, CCK, PYY, PP, amylin, insulin, GIP, and ghrelin.
What makes sustained weight loss challenging is that when you lose a significant amount of weight, your hormones change, which can lead to the activation of the group that leads to weight gain and the inhibition of the group that leads to weight loss. To make things even more challenging, these neurons can be damaged with weight gain such that they are less sensitive to these hormones.
When you gain weight, it becomes increasingly difficult to lose it as your body undergoes physiological changes favoring weight gain. If you lose significant weight, your brain increases your appetite and works to decrease energy expenditure.
Side note: Unfortunately, we can’t inject people with massive quantities of leptin to decrease their appetite and solve obesity. When we first discovered leptin in the early 1990s, we thought we might just have the cure to obesity. This only works in the extremely rare cases where people have a leptin deficiency (they are basically always starving). In those cases, we can cure their obesity and regulate their appetite with exogenous leptin. However, in most cases, obesity is not caused by a leptin deficiency. In fact, people with obesity typically have high leptin levels. This led researchers to conclude that leptin resistance is part of the problem, not leptin deficiency, which shows just how complex this science is.
How does this system lead to weight gain?
The lipostat system is asymmetric. It protects far more against weight loss than weight gain. Imagine a thermostat that is very easy to turn up but really hard to turn down.
The set point your body is trying to defend tends to creep up as we gain weight. The higher weight becomes the new normal the body tries to defend (the new “ideal” that the lipostat system has in mind).
This is why we see rebounds after weight loss from diets. A person who previously lost weight is much more susceptible to weight gain because their brain is fighting to regain that fat. The brain initiates a starvation response and engages in a suite of behavioral and physiological responses intended to increase one's calorie intake and, at times, it will even curtail your metabolic rate. There is also an unconscious decrease in physical activity although increased appetite is considered a much more important factor.
This is all in service of gathering more fat in your fat tissue to align with the amount of body fat your brain wants you to have.
The problem today is it’s too easy to find calorie-dense food that has been engineered for peak deliciousness (what you might hear called “the bliss point”) and the exact thing our brains have been wired to crave, and have been rewarded for acquiring, for thousands of years. It’s understandable we’re having a hard time managing. In fairness, the current reward-based motivational system has carried us pretty far.
Any animal will gain weight when exposed to a food in the following ways:
Wide variety: our brains recognize variety as potentially additional nutrients that we may not have consumed yet. This is the same reason you always “have room for dessert.” Side note: making meals boring can decrease energy intake.
Easy access: Friction matters. Do you have to cook food (we did before refrigerators and DoorDash)?
Unlimited (ad infinitum): Most people in developed countries do not experience calorie shortages and have access to food ad infinitum (removing quality).
Tasty: Most people would eat far less if they had to eat what people ate 1000 years ago (even if they had access to it in abundance). It just didn’t taste that good.
Simply put, there is an evolutionary mismatch between your brain and the current environment. Not to mention, we’re still learning how our brain works and our understanding of the above will surely evolve in the coming years.
We shouldn’t treat people with obesity with drugs, shouldn’t we just fix the food we eat instead?
We need both.
One of the biggest distinctions needed in the discourse about obesity is the clear distinction between “Obesity Prevention vs. Obesity Treatment,” the techniques available to accomplish both, and the data currently supporting those techniques.
Dr. David Allison said it extremely well on Peter Attia’s Podcast. “Wouldn’t it be better if we had a world where no one needed surgery? Where our solution to obesity is not one in which we let children gain weight and then when they become adults with obesity give them surgery and re-arrange their internal anatomy. Yes. That would be better. But that’s not the world we live in right now.”
The point he makes is that we need to both prevent future obesity and to treat current obesity to reduce suffering — and confusing the two can prevent us from caring for the people who need help today. The reason the distinction is so important is because, while there is overlap, the most effective techniques to treat obesity are different from the techniques to prevent obesity.
For example, a healthy diet and frequent exercise are phenomenal tools at preventing obesity. They are even vital complements to the medical treatment of overweight and obesity. But, as discussed above, the data do not show these tools alone driving significant enough and sustainable weight loss at the population level for many individuals (again, diet and exercise are amazing for overall health regardless of its impact on weight).
As a result, telling people who have overweight or obesity to “diet and exercise” alone to treat obesity will be ineffective just as providing medication without lifestyle changes would be insufficient. As mentioned above, they’ve undergone physiological adaptations that favor weight gain, making it much harder to lose weight after the fact than maintain a healthy weight from the outset (e.g., prevent vs. treat).
Dr. Lou Arrone put it best when he said — “Many obesity-related health problems are worsened by circumstances that could be helped through policy—by raising the minimum wage high enough for people to afford fresh produce and high-quality protein, by investing in housing and community spaces that are conducive to recreation, by ending the billions of dollars in farm subsidies that go to junk-food additives, such as high-fructose corn syrup. These things would work to prevent obesity, not treat it. It would be like trying to treat lung cancer through a smoking-cessation program.”
In addition to needing to make the distinction between prevention and treatment, we also need to be unbiased and fact-based about the current techniques that have proven to be effective at both.
It is abundantly clear that we are currently horrible at preventing obesity at the population level, across the globe. In 2014, the New York Times published an article titled — “No Nation Has Lowered Obesity Rate in 33 Years.” Our public health initiatives, community initiatives, school-based initiatives, and our overall policy-based approach has failed. This does not mean we shouldn’t keep trying. But we must acknowledge our failure. The reason we need to acknowledge our failure is because, if we’re failing, we cannot continue to do the exact same thing and expect different results.
Breaking it down further:
Prevention: We’re currently failing. We don’t have great examples of interventions that have worked at the population level (only individual level).
Treatment: We’re currently failing. We have strong evidence of interventions that are successful, an example of which is surgery (which is inaccessible to most). But GLP-1s do represent (cost aside for now) a potentially scalable and effective treatment for obesity.
Denying the majority of the population treatment to reduce suffering, reduce healthcare costs, and improve their lives because we wish the food supply chain were different or we didn’t have corn syrup subsidies is ignoring the fact that we need to solve two distinct problems where the data have shown us that only some, not all, of the solutions overlap.
We must work to prevent obesity for current and future generations. We must work tirelessly to protect children from the negative impact of poor diet, lack of exercise, and excess weight.
AND
We must work tirelessly to treat the people we may have failed to this point. We must treat the people suffering today. Fortunately, we’re starting to have increasingly effective and scalable tools. Now, we must reduce the cost and democratize access.
Isn’t obesity just a disease of the poor and uneducated?
You often hear people say, “obesity is a disease of the poor and uneducated.”
This is a gross oversimplification and, when taking into account different demographic groups, it is not accurate. For example, while income has some correlation with obesity for white women, this correlation is far more tenuous for Black women. Understanding the data can help us understand that the causes of obesity for many people reflect far more than income and education level.
“The association between obesity and income or educational level is complex and differs by sex, and race/non-Hispanic origin.” — CDC in “Prevalence of Obesity Among Adults, by Household Income and Education — United States, 2011–2014”.
As it relates to income, according to the CDC, we see:
Women: The prevalence of obesity decreased with increasing income in women (from 45.2% to 29.7%)
Men: There was no difference in obesity prevalence between the lowest (31.5%) and highest (32.6%) income groups among men.
As it relates to education:
Obesity prevalence was lower among college graduates than among persons with less education for:
Non-Hispanic white women
Non-Hispanic white men
Non-Hispanic black women
Hispanic women
Obesity prevalence was not lower among college graduates than among persons with less education for:
Non-Hispanic Asian women
Non-Hispanic Asian men
Non-Hispanic Black men
Hispanic men
Obesity based on race (CDC NHANES):
ADULTS: Prevalence of adults aged 20 and over with obesity, by demographic characteristics: United States, 2017–March 2020
Race & Hispanic Origin | All Sexes | Male | Female |
Non-Hispanic Black | 49.9% | 40.4% | 57.9% |
Hispanic | 45.6% | 45.2% | 45.7% |
Non-Hispanic White | 41.4% | 43.1% | 39.6% |
Non-Hispanic Asian | 16.51% | 17.6% | 14.5% |
CHILDREN: Prevalence of children and adolescents aged 2–19 years with obesity, by demographic characteristics: United States, 2017–March 2020
Race & Hispanic Origin | All Sexes | Male | Female |
Non-Hispanic Black | 24.8% | 18.8% | 30.8% |
Hispanic | 26.2% | 29.3% | 23.0% |
Non-Hispanic White | 16.6% | 17.6% | 15.4% |
Non-Hispanic Asian | 9.0% | 13.1% | 5.2% |
Not everyone with a higher BMI is metabolically unhealthy. How do you reconcile that?
Yes, BMI is a crude metric. It has its limitations, but it isn’t useless. It’s just not perfect in all circumstances (a high bar for any measurement). Metrics should be fit for purpose, and BMI absolutely has a valuable purpose.
In a perfect world, with unlimited resources and unlimited time, at the patient level, we’d look at more granular clinical markers of obesity (e.g., visceral fat, bone mineral density, muscle mass index) beyond BMI. These clinical markers can be determined with lab testing and expensive imagery. To determine your BMI, you need your height in meters squared and your weight in kilograms. BMI is free to calculate whereas the ideal clinical markers can cost in excess of $1000 (can cost, not should cost).
So when and why do we use BMI?
You often hear “But Lebron James would be overweight based on the BMI scale.” That’s true. But the majority of people are not Lebron James. While not perfect, BMI is a good proxy for percentage of body fat at the population level. Here is a study showing the high correlation between body fat % and BMI for men and women (link to figure below). Note: the body gat % in these graphs were measured by bioimpedance, not a Dexa scan.
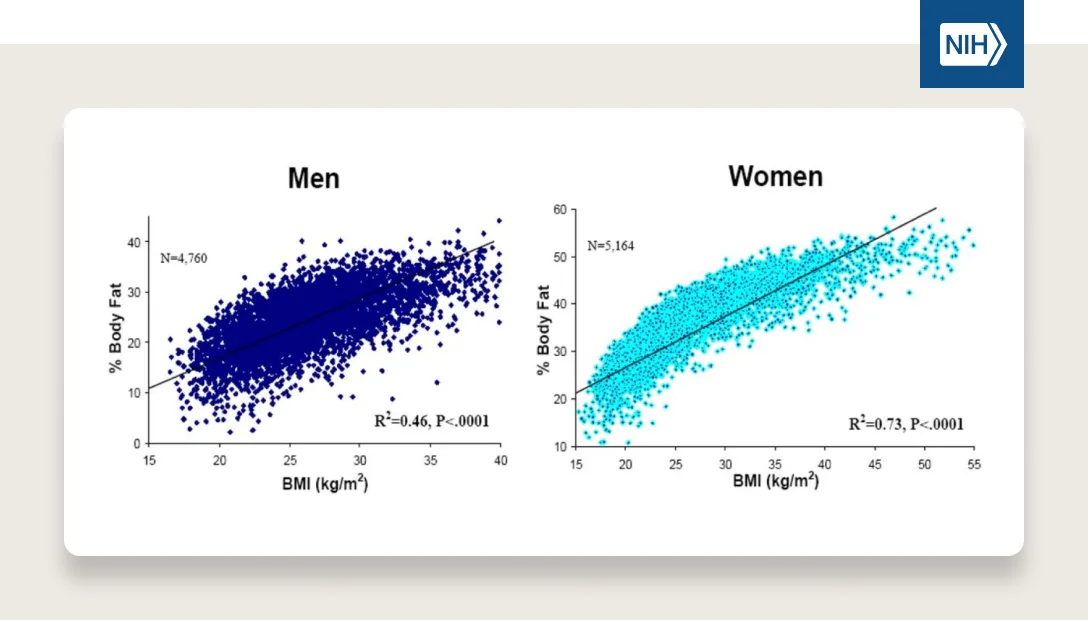
Because BMI is highly correlated with body fat % and, as mentioned above, it’s very easy to calculate and it’s free, we can use it to study populations over time more easily. We’ve been measuring soldiers’ heights and weights for over 100 years, which is one of the ways we are able to track transitions in body composition over time.
While not everyone with a high BMI is metabolically unhealthy, it is still a good proxy for us to understand the health of a population.
The majority of people with high BMI are in fact metabolically unhealthy
A meta analysis on “The prevalence, metabolic risk and effects of lifestyle intervention for metabolically healthy obesity: a systematic review and meta-analysis” estimates ~65% of patients with obesity were metabolically unhealthy.
Even for those who are metabolically healthy, for the majority of people, time at a higher BMI puts someone at increased risk for advancement to an unhealthy state and adverse events.
Of the 35% who were metabolically healthy, they had a greater chance of becoming metabolically unhealthy and “they are still at higher risk of advancing to unhealthy state. “Therefore, it is still needed to advise MHO [Metabolically Healthy Obese] individuals to maintain or adopt a healthy lifestyle, so as to counterbalance the adverse effects of obesity”.
An analogy here that might be helpful is thinking about this similar to smoking cessation. Imagine asking the question “Do you currently smoke?” Someone who has smoked for 2 weeks, and another who has smoked for 20 years, would each answer yes. But they likely have different health outcomes based on the time they spent smoking.
Even fewer people with a BMI of 35+ are metabolically healthy
Even though BMI is not a perfect measurement of metabolic health, research shows that increased BMI is associated with poorer metabolic health. For example, a recent study showed in all years from 1999-2018, fewer than 15% of adults were considered metabolically healthy once their BMIs exceeded 34.9. For BMIs > 39.9, fewer than 9% of subjects were considered to be metabolically healthy.
Noteworthy regarding BMI: the starting point at which people might experience metabolic disturbances can vary by race and sex. For example, Asian Americans can have higher rates of metabolic disturbances at lower BMIs. This is another example of BMI’s limitations.
TLDR:
BMI is a great proxy for population health as it is highly correlated with body fat % and is free and easy to collect.
BMI should not be the end-all-be-all for determining whether an individual is at an appropriate weight and determining what the best treatment is for an individual. There are superior clinical markers, but they can be expensive to collect.
Unfortunately, given the role BMI plays within our insurance-based healthcare system and academic research, it will be extremely hard to unwind its role at the individual level in the near future.
A “healthy BMI” can vary by race and sex.
What about the Obesity Paradox showing that higher BMIs are actually beneficial for certain causes of death?
You’ll often hear people say, “having a higher BMI can actually be more protective in old age” or they will reference the “obesity paradox.” Holding the fact that it’s not really a paradox aside, these are the data to which they are referring:
SOURCE: THE LANCET
You’ll see these graphs show a concave up curve, with the nadir around 25–27 (depending on the graph). This would imply that people with a BMI currently classified as “overweight” have better health outcomes when just looking at BMI at death. Given that the negative health effects of excess adiposity are strongly supported by data, the conclusions that are drawn from this graph are surprising (hence “the obesity paradox”).
So, what do we make of this “obesity paradox.” Evidence is building that “the ‘obesity paradox’ is a mirage caused by confounding in observational studies.” The meta analysis for BMI-mortality association (Sources and severity of bias in estimates of the BMI–mortality association) highlights three important biases that likely exist in survey-based estimates of BMI-mortality association:
Residual confounding bias via within-BMI-group variation in body shape
Positive survival bias in high-BMI samples due to recent weight gain (variation in duration of time with obesity)
Negative survival bias in low-BMI samples via recent illness related weight loss
Quick translation of the biases above:
#1 says that there is some variation in body composition that is perfectly correlated with BMI (what we discussed above)
#2 says that there are circumstances where someone has a lower BMI and is healthy but gains weight quickly just before the point of measurement and has not been at a high BMI for a long enough period of time to experience the negative effects of excess weight (pushing the average healthy BMI up).
#3 says that certain illness may cause severe weight loss just before death (e.g., HIV, kidney disease, cancer) such that if you measure BMI at death it will be artificially low making it appear that a lower BMI is “unhealthy” (i.e., pushing the “healthy BMI up”)
Basically, what the two latter biases seem to describe is the “obesity paradox” as the result of confounding variables because the measurement of BMI is a static measurement in these surveys (i.e., an image instead of a movie). The movie tells a different story.
What happens when we start to account for length of time that someone is at a particular BMI, we start to tease apart the confounding variables and see evidence from this study indicating that all “three sources of variation influence the health composition of BMI samples in the NHANES data and downwardly bias estimates of the BMI–mortality association in the US. Results from survival analyses that adjusted for these biases suggest that overweight and obese BMI levels strongly elevate mortality risk in the US adult population.”
Meaning, the three biases described above shift the BMI-association with mortality when looking at it as a static picture instead of over a longer term time horizon. Let’s take a look at what happens over a 10 year time horizon, when patients start either having obesity or not. We see that over 10 years, they can fall into 1 of 5 Groups.
SOURCE
When these groups measure their BMI 10 years later, we start to see a story play out. When we adjust the model for a longer time period, we start to see the “obesity paradox” disappear. All-cause mortality correlates with extremely low BMIs and BMIs that fall under the current definition of overweight and obesity.
SOURCE
The basic point here is that while bodies are different and average healthy BMIs can vary by sex, age, and race, as confounding variables are teased apart, more evidence strongly suggests that having a higher BMI (or having excess adiposity) for extended periods of time is highly associated with negative health outcomes.
Now, of course, there is nuance here. For instance, there is also evidence suggesting that excess weight in certain circumstances can indeed be protective or beneficial. For example, if you fall and land on your hip, you’d rather have some excess weight than not. And there are certain circumstances when you’re in the hospital (e.g., X infection) where it could be helpful to have excess weight. But, with excess weight, falling in the first place or ending up in the hospital might be more likely. As I said, it’s nuanced.
Shouldn’t we just tell people to diet and exercise?
From a health perspective, there is no substitute for diet and exercise. Both are phenomenal for your overall health — healthspan and lifespan. They are also amazing tools to prevent (or attenuate) weight gain. But the practical reality is that they have not been shown to lead to significant and sustainable weight loss at the population level.
Exercise
Dr. Peter Attia puts it well, “Physical exercise is one of the best ways to maintain overall health with age. Exercise is essential for increasing aerobic capacity, cardiac output, and insulin sensitivity. Exercise is also an effective means to improve cognition and mood while lowering stress.”
In terms of exercise in service of weight loss, there are two categories:
Supervised exercise
Unsupervised exercise
Supervised exercise has been shown to lead to 5–8% weight loss in specific studies. Supervised exercise is exactly as it sounds. People are supervised when they exercise, and it is usually fairly intense. The studies that show 5–8% weight loss result from exercise programs that are typically 5 days per week, 1 hour long exercise routines. These exercise programs require people to burn 500–700 kcal per session. As mentioned above with regards to a patient's “set point,” the majority of people regain the weight when they stop exercising as vigorously. For those who are able to maintain this routine, they can sometimes maintain their lower weight — but the routine described above is hard to maintain.
We don’t see nearly the same efficacy with unsupervised exercise.
Diet:
This is a subject with zealots on either end of the spectrum in the scientific and medical community, with very few in between. There are those who believe nutritional epidemiology is a dead science and we should do away with it, and those who defend the status quo with incredible vigor and think anyone who criticizes it is an idiot. The truth, unsurprisingly, is likely somewhere in the middle.
It is true that:
Many nutritional studies have not held up to the scrutiny of randomly controlled trials.
President Taft’s physician, Dr. Nathaniel Yorke-Davies, wrote him letters in 1905 that read as if they could have been written today — “The diet Yorke-Davies prescribed specified lean meats, a little fish, vegetables, plain salad, avoidance of sugar, and minimal carbohydrates.” Not as much as you’d think has changed in the last 100 years.
We have absolutely no idea if eating a hot dog takes 36 minutes off your life.
Asking people exactly what they ate from a food questionnaire is useless.
On the other hand, we know that we didn’t have obesity or nearly the same rates of Type 2 diabetes 100 years ago. We also know the American diet, sometimes known as the “cafeteria diet,” is not good for our health and is one of the fastest and most efficient ways to gain weight (in rats and in humans).
In summary:
Diet and exercise are important for your overall health.
They can be great tools to prevent or attenuate weight gain (remember the important distinction between prevention and treatment described above).
They are not generally linked to significant and sustainable weight loss — in part because they are extremely difficult to follow — we don’t have perfect knowledge of what works, and our body will often fight us in maintaining the weight loss (i.e., stimulate a starvation response).
This is where GLP-1s can be profoundly helpful. GLP-1s can make it easier for many people to follow a diet (not replace the need for a diet). The medications will fight the body’s starvation response and make it easier for people to defend a lower set point. The easier a diet is, the more people see success, the more likely they are to stick to it (we see this in behavioral health studies as well — success leads to greater adherence). Think of a GLP-1 agonist like a diet adherence jetpack — just making it easier.
When people come to Ro for help with obesity, they often have stories about the diet or exercise solutions they have tried, about continuous hunger that prevents them from sleeping, or exhaustion from trying so many times so many methods to keep weight off that doesn’t work. For people who have not struggled with the chronic disease that is obesity, “diet and exercise” might sound like a simple solution. It is not.
On the exercise front, it’s vital for long term health and especially vital if people are experiencing weight loss. As mentioned above, patients who lose weight will see some portion of lean muscle mass loss. Now, people who have overweight are often carrying a bit of excess lean mass to begin with, but too much lean muscle mass loss isn’t good. Exercise (particularly resistance training) can help attenuate lean muscle loss as someone loses weight on a GLP-1 (or through other interventions). Adequate protein intake is also important for decreasing lean mass loss when losing weight.
Isn’t this problem mainly in America?
There is a perception that “America is fatter than other countries.” While the US does have higher rates of overweight and obesity, other countries are not far behind.
As discussed above, obesity is an externality to society solving scarcity. The US is clearly above other countries in terms of obesity rates, but other countries are seeing their rates rise at similar rates (the US started above the pack in 1975 as well). The US got rich first.
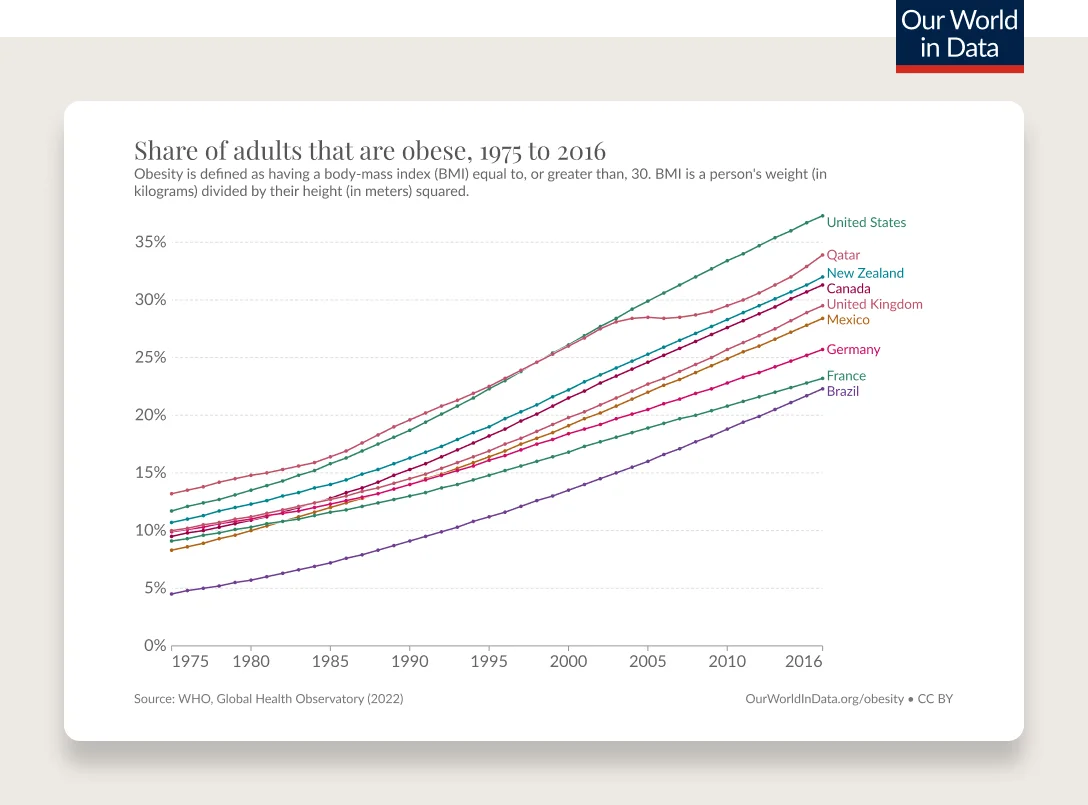
SOURCE: OUR WORLD IN DATA
The lines get even closer together when we look at overweight and obesity. Meaning, other countries' overweight rates are increasing a bit faster. But if we play this movie forward, those people are on a path to have obesity as well. Note: these rates are all higher today (the data goes to 2016, the US is closer to ~76% in 2023).
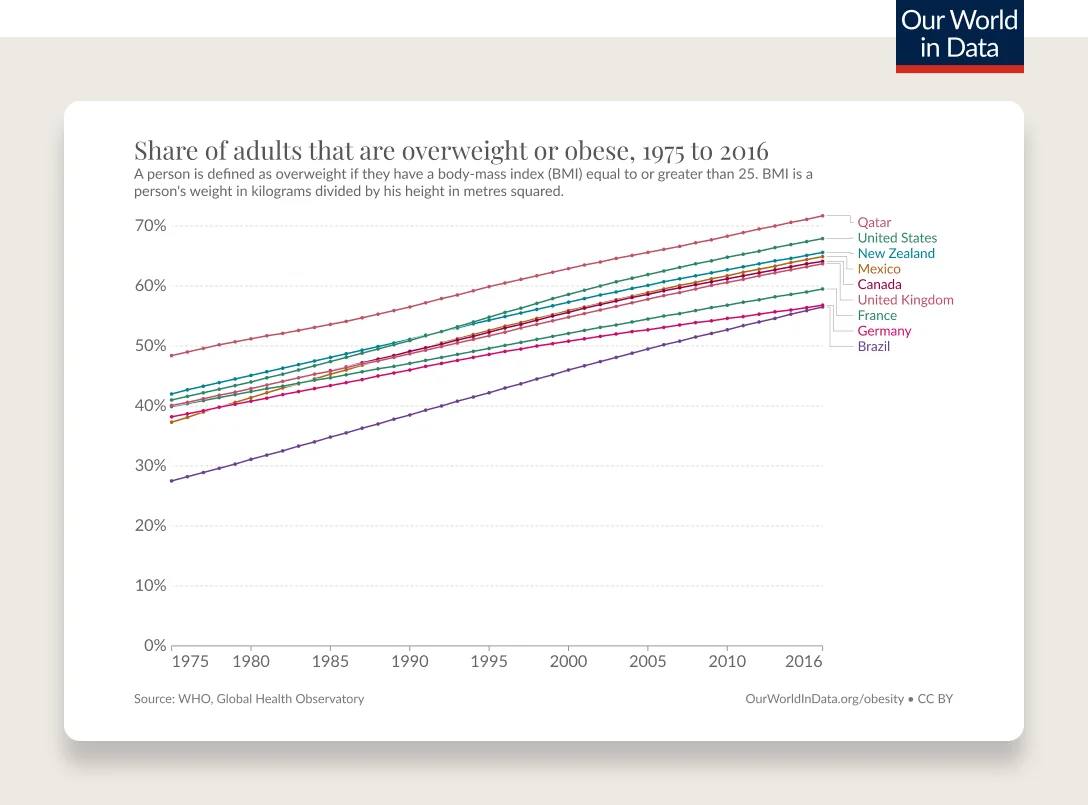
SOURCE: OUR WORLD IN DATA
The countries with the fastest growth rates, versus absolute highest rates, are the ones that have lifted their countries out of poverty over the last 30 years. China’s relative growth rate of individuals with a BMI of greater than 25 since 1975 is 226% versus 66% for the US. And its absolute growth rate is similar to the US over the last 40 years (+22.40 pp for China versus +26.90 pp for the U.S.). Also, as mentioned above, “the starting point at which people might experience metabolic disturbances can vary by race and sex”. This adds an additional layer of complexity when analyzing BMI by country as certain BMIs may be correlated with worse health from a metabolic standpoint in certain populations.
Are there exceptions? Is it perfectly correlated with GDP or economic growth?
There are a few exceptions. But they are the minority. Japan is one of them.
SOURCE: OUR WORLD IN DATA
But breaking down Japan even further, you see the average is more so driven down by the BMI of women shrinking over time, which is astounding (note: the BMI of men is still increasing over time, just not nearly as drastically as the US).
SOURCE: OUR WORLD IN DATA
For the most part, this is a global issue. The US is just about 20 years “ahead”.
Will obesity treatment cause an increase in eating disorders and binge eating in adults with overweight or obesity?
Obesity care should be delivered with sensitivity to each individual’s relationship with food, body image, and consumption. Looking at the data available, a recent systematic review with meta-analysis was published recently (March 2023) on “Eating disorder risk during behavioral weight management in adults with overweight or obesity.” Dr. Michael Albert (obesity expert with an extremely insightful Twitter account) describes the takeaways from the study well. “People should be stratified and monitored on an individual level. Still, generally, behavioral weight management interventions don’t increase the risk of eating disorders and may reduce binge eating risks.” While this research did not evaluate obesity treatment with GLP-1 medications, it shows that there is evidence to suggest that weight management interventions in adults with overweight and obesity have a positive impact. It will be important for further research to study this topic as it relates specifically to treatment with GLP-1 medications.
Why is there so much vitriol in the conversation about obesity?
As I mentioned at the start of this Q&A, it’s deeply personal. It’s influenced by our cultures, family, commerce, politics, philosophy, ethical considerations, sustainability beliefs, our childhood, and more. We eat every day, and it’s very frequently perceived as a reflection of some part of our identity.
Also, we weren’t always in the place we are today. There are strong financial incentives (e.g., food subsidies of corn syrup) that make it increasingly difficult to prevent obesity. Look no further than “Fruity Pebbles and Lucky Charms threaten to block ‘healthy’ food labeling guidelines in court.” The FDA’s proposed labeling requirement is facing backlash from processed food companies claiming the rule “limits their free speech.” We are fighting an uphill battle, and it can be incredibly frustrating because we created the obesogenic environment we live in today (not intentionally but through the incentives we’ve created). People are justified in their frustration.
This is a hard topic. One with which the majority of the US population has had intimate and first-person experience. It’s hard if their experience differs from aggregate data or vice versa. This isn’t an easy topic to discuss, to debate, let alone to solve.
Whenever you see anger (versus genuine intellectual curiosity and debate) in a Twitter thread or TikTok video about GLP-1s, nutrition, obesity rates and causes, try to imagine why that person has such anger in their heart about this topic. It’s most likely because they, or someone they love, has struggled with overweight or obesity and has received incorrect, insensitive, or insufficient advice and guidance. When we are tired (truly exhausted), our heart shrinks. Remember that whenever engaging on the topic.
Last but not least, this problem is multi-factorial. For the majority of people with overweight or obesity, there is no single cause and easy fix.
Shouldn’t we celebrate body positivity? Doesn’t this medication and conversation reinforce fat shaming?
Dr. Rachel Goldman, a psychologist and NYU Clinical Assistant Professor, has been a tremendous source of education on this topic for me.
She explained the idea that we should shift away from the term “body positivity” and toward one of “body neutrality.” Dr. Goldman says, “It can be quite harmful to present body acceptance the way that body positivity does because it may not necessarily be possible to attain this kind of acceptance. Not much in life is that ‘all or nothing,’ and it’s quite difficult for us to ‘love’ our body all the time. It’s normal, and okay, to not fully love our body all the time. That is where body neutrality comes in. It’s getting away from this idea that we have to love our body. Instead, we can just focus on accepting it for what it is, and what it does for us.”
Another way “body neutrality” was described to me that I found particularly powerful was the following. Dr. Tzvi Doron, a board-certified obesity physician, Ro’s former Chief Clinical Officer, and a friend of mine, wrote “body neutrality means we don't get our self-worth from our bodies in the first place. Imagine you have some other illness like rheumatoid arthritis. Should that cause you to hate yourself? No, because you know that you simply have an illness. It doesn't make your body great or terrible, and it definitely doesn't make you great or terrible. It just means you have something to manage.”
While the concept of “body positivity” comes with the best of intentions, it’s an unreasonable expectation for everyone to be positive about their body all the time. We’re expected to go from potentially struggling with our own body image to loving our bodies no matter what. This unreasonable expectation has created the potential for another perceived “failure” and sets people up for unfair disappointment.
In terms of steps we can take to shift our mindset, Dr. Goldman recommends a few things:
Remind yourself of what your body is capable of (what it does for you).
Wear clothes that fit and make us feel good — “dress to impress you.”
Be mindful of who you follow on social media. Unfollow, mute, or block anyone that doesn’t make you feel good or makes you question your own worth.
One thing I want to call out is that this is genuinely hard to talk about. Experts are identifying the potential negative consequences of using the terminology “Body Positivity,” a term that I do not think we would have predicted would have the negative consequences it has had for some people. It’s clear we’re all learning together and we’d benefit from far more empathy as we find the right language that helps the greatest number of people while causing the least amount of harm. It’s not obvious, it’s not easy, but we can get there.