Important safety information
What you should know before taking GLP-1s.
On-demand provider coaching and support
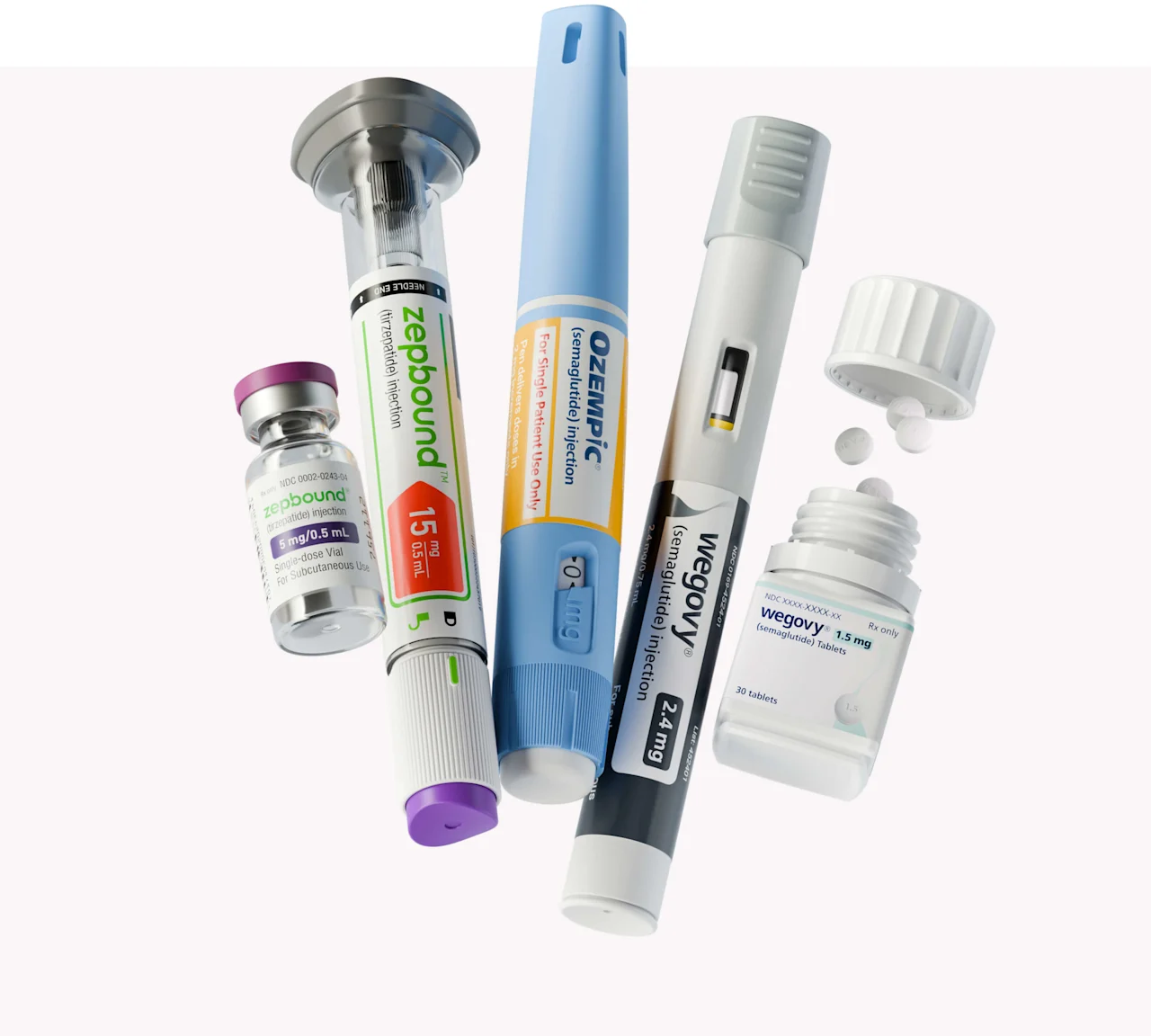
More GLP-1 options to fit your needs
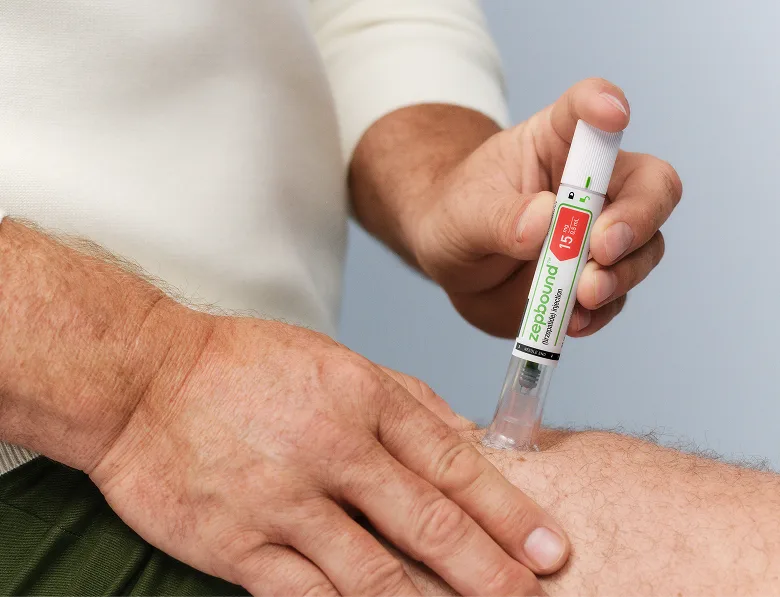
Helps you feel full faster, longer
Safety info: GLP-1 medications may have serious side effects, including possible thyroid tumors. Do not use if you or your family have a history of a type of thyroid cancer called MTC or MEN 2. See more serious warnings & safety info here.
Select your current weight
252lbs
Weight you could lose (lbs):
50lbs
*In separate 68- and 72-week clinical trials studying Wegovy (2.4 mg) and Zepbound (15 mg) in patients without diabetes and with obesity, or overweight with a weight-related condition, the average weight loss was 15% and 20%, when paired with diet and exercise changes (compared to 2.4% and 3.1%, respectively, with diet and exercise alone).
Ozempic is FDA-approved for type 2 diabetes treatment, but may be prescribed off-label for weight loss at a healthcare provider’s discretion.
From personalized treatment to insurance support, we help you get—and stay—on track.
Answer a few questions about your health and goals, all online—no in-person doctor’s office visit necessary. You’ll find out if you’re eligible within 2 days.
A provider will review your info and prescribe the right GLP-1 treatment for you, if eligible. Get ready for your first dose in two weeks if using insurance, or less than a week if paying cash.
Ro checks your insurance to see if GLP-1 coverage is available. If you require prior authorization, Ro's insurance concierge fights for coverage and submits all paperwork on your behalf. If you're covered, a prescription is sent to your pharmacy. If not, your provider will suggest alternative FDA-approved cash pay GLP-1 options. This process takes about 2-3 weeks.
If paying cash, your medication ships right to your door and you can take your first dose in less than a week. If using insurance, your medication can be picked up at your pharmacy.
Keep making progress with regular check-ins, expert guidance, and access to your care team 24/7. Ongoing care and support is there for you when you need it—whether it’s a quick chat with your provider or routine check-ins, we’re with you every step of the way.
Thousands of members and counting
Don't just take it from us—our members see (and feel) the difference. These members were paid in exchange for their testimonials.
87% have seen life-changing results*
93% agree Ro is easier to incorporate into their lives*
97% report silenced or quieter food noise since starting*
Average weight loss in 1 year is 14-20% (vs 2.2-3.1% with diet and exercise alone). Based on studies of branded medications in non-diabetics with obesity, or with overweight plus a weight-related condition, with diet and exercise.
*Based on survey results of 1,243 Ro members taking GLP-1 medication for at least 7 weeks, paired with diet and exercise.
Meet our expert
Feel like you’re doing everything right, but the weight still doesn’t budge? It’s not just a matter of willpower. Our board-certified expert, Dr. Steve, explains the biology behind why your body might need more to get the results you want.
Ro members were paid for their testimonials.
Average weight loss in 1 year is 14-20% (vs 2.2-3.1% with diet and exercise alone). Based on studies of branded medications in non-diabetics with obesity, or with overweight plus a weight-related condition, with diet and exercise.
*Based on survey results of 1,243 Ro members taking GLP-1 medication for at least 7 weeks, paired with diet and exercise.
The Ro Body membership costs $45 for your first month, then $145 thereafter.
Please note that the cost of GLP-1 medication is not included in the membership cost. Medication cost will depend on your treatment and personal insurance coverage.
Ro’s insurance concierge will work with your insurance provider to determine coverage for your GLP-1 medication. Please note that this medication is paid for separately from your Ro Body membership, which is only available by cash pay only and does not accept insurance.
Currently, Ro can’t help coordinate coverage for GLP-1 medications for government insurance plans — but depending on your plan, you may still be able to pay out of pocket for certain cash-pay medication options. If you have Federal Employee Health Benefits Program (FEHB), you can join the Ro Body membership and access our insurance concierge.
If your provider orders a metabolic health test, testing at any Quest location is included in the cost of the Ro Body membership. Or you can purchase an at-home blood collection kit through Ro for $75.
If you live in a state where Quest is not available, we’ll automatically send you an at-home collection kit for no charge.
If for some reason your insurance will not cover the cost of your medication, you’ll have the option to either select from cash pay medication options or you can cancel your Ro Body membership at any time.
After you complete your online visit, a Ro-affiliated healthcare provider will review your answers and determine whether treatment may be right for you. Depending on your health review, your provider may ask you to take a metabolic lab test. If appropriate, your provider will discuss GLP-1 medication options and recommend a personalized treatment plan.