Important safety information
What you should know before taking GLP-1s.
On-demand provider coaching and support
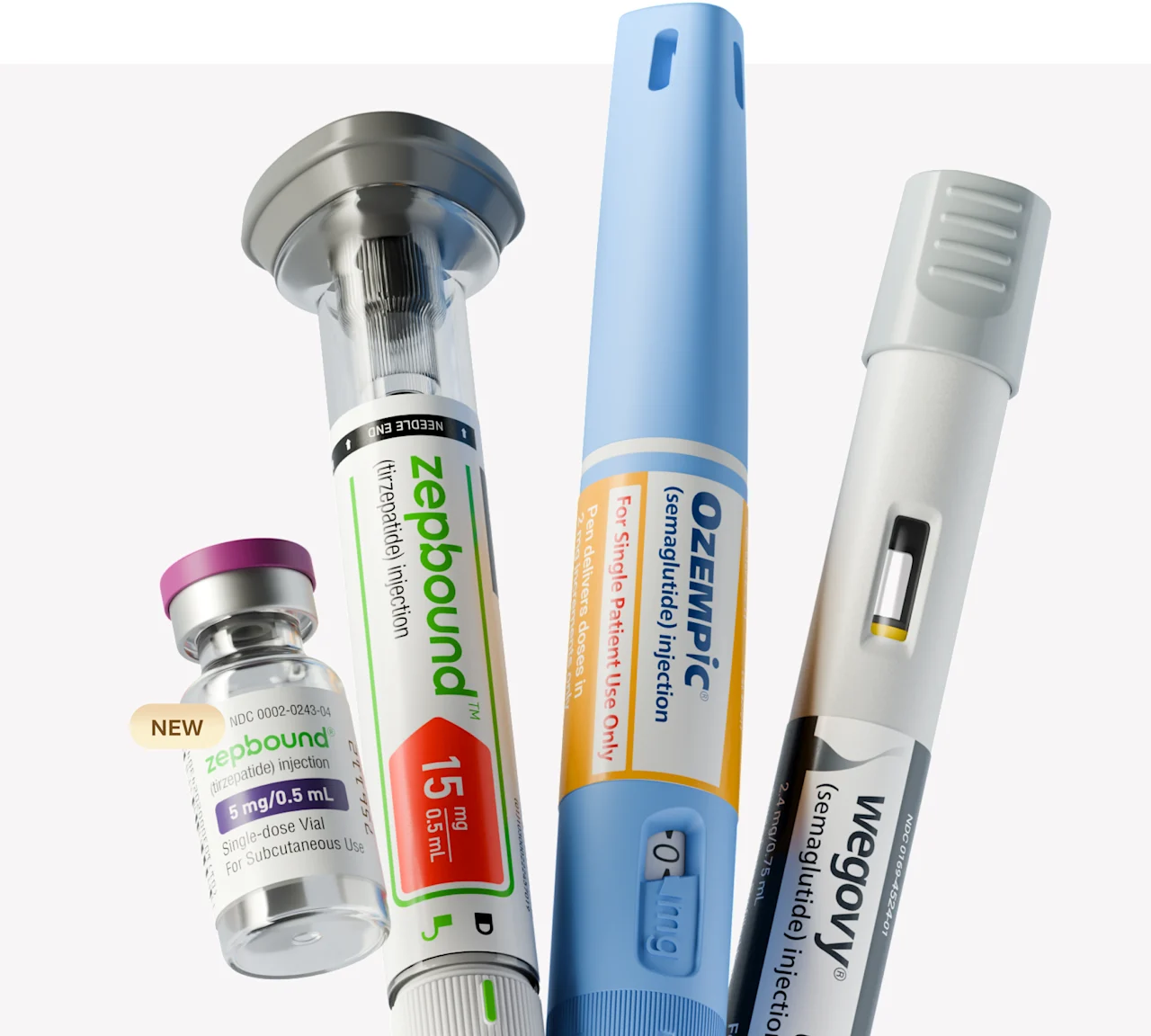
More GLP-1 options to fit your needs
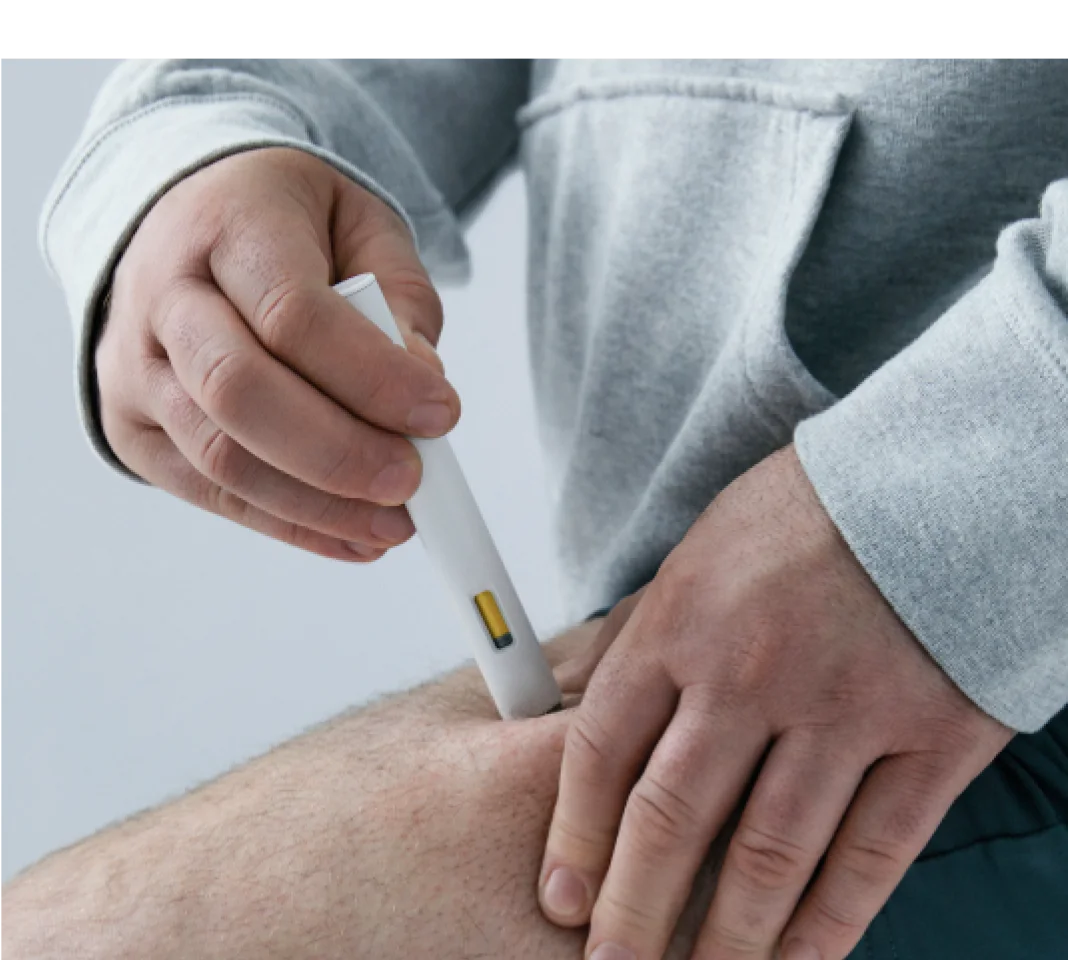
Helps you feel full faster, longer
Safety info: GLP-1 medications may have serious side effects, including possible thyroid tumors. Do not use if you or your family have a history of a type of thyroid cancer called MTC or MEN 2. See more serious warnings & safety info here.
Select your current weight
252lbs
Weight you could lose (lbs):
50lbs
*In a 68- and 72-week clinical trial studying Wegovy (2.4 mg) and Zepbound (15 mg) in patients without diabetes and with BMI ≥30, or BMI ≥27 with a weight-related condition, the average weight loss was 15% and 20%, when paired with diet and exercise changes (compared to 2.4% and 3.1%, respectively, with diet and exercise alone).
Zepbound and Wegovy are FDA-approved for weight loss. Ozempic is FDA-approved for type 2 diabetes treatment, but may be prescribed off-label for weight loss at a healthcare provider’s discretion.
Average weight-loss 15-20% in one year. Based on studies of branded medications in non-diabetics with obesity, or with overweight plus a weight-related condition, when paired with healthy lifestyle changes.
Whether you want to check your insurance coverage or prefer to pay cash, we got you. Once you’re a member, we take care of any paperwork and insurance back-and-forth so you don’t have to lift a finger.
Share your health history and weight loss goals with us online to get started. A Ro-affiliated provider will review your answers and get back to you within a few days. Depending on your health review, a provider may order a metabolic lab test before moving forward with treatment.
After reviewing your results, your provider will determine if you’re eligible for medication. If you are, they’ll tailor a program with prescription treatment best suited to your unique biology.
If you’d like to use insurance, a dedicated team will connect with your insurance company to determine your coverage. If you’d prefer to pay cash or if coverage is denied, we’ll help you understand your options across our full GLP-1 suite. Zepbound vials cannot be purchased with insurance, and are cash pay only.
If you’re paying cash for your medication, it will typically be shipped to you in 1-4 days. If using insurance, you’ll get your prescription from your preferred pharmacy. Medication costs are not included in Ro Body membership pricing.
You’ll have everything you need, including on-demand provider messaging, personalized medication adjustments, and expert guidance on what to expect on your journey – all as part of your membership.
Thousands of members and counting
Don't just take it from us – our members see (and feel) the difference. These members were paid in exchange for their testimonials.
87% have seen life-changing results
93% agree Ro is easier to incorporate into their lives
97% report silenced or quieter food noise since starting
Based on survey results of 1,243 Ro members taking GLP-1 medication for at least 7 weeks, paired with diet and exercise.
Average weight loss in 1 year is 15-20% (compared to 2.4-3.1% with diet and exercise alone). Weight loss results based on studies of branded medications in non-diabetics with BMI ≥30, or BMI ≥27 plus a weight-related condition, when paired with healthy lifestyle changes.
Meet our expert
When it comes to weight loss, biology is your nemesis. Not willpower. Our very own Dr. Steve explains why.
Ro members taking branded GLP-1 medications were paid for their testimonials.
The Ro Body membership costs $45 for your first month, then $145 thereafter.
Please note that the cost of GLP-1 medication is not included in the membership cost. Medication cost will depend on your treatment and personal insurance coverage.
Ro’s insurance concierge will work with your insurance provider to determine coverage for your GLP-1 medication. Please note that this medication is paid for separately from your Ro Body membership, which is only available by cash pay only and does not accept insurance.
Currently, Ro can’t help coordinate coverage for GLP-1 medications for government insurance plans — but depending on your plan, you may still be able to pay out of pocket for certain cash-pay medication options. If you have Federal Employee Health Benefits Program (FEHB), you can join the Ro Body membership and access our insurance concierge.
If your provider orders a metabolic health test, testing at any Quest location is included in the cost of the Ro Body membership. Or you can purchase an at-home blood collection kit through Ro for $75.
If you live in a state where Quest is not available, we’ll automatically send you an at-home collection kit for no charge.
If for some reason your insurance will not cover the cost of your medication, you’ll have the option to either select from cash pay medication options or you can cancel your Ro Body membership at any time.
After you complete your online visit, a Ro-affiliated healthcare provider will review your answers and determine whether treatment may be right for you. Depending on your health review, your provider may ask you to take a metabolic lab test. If appropriate, your provider will discuss GLP-1 medication options and recommend a personalized treatment plan.