Key takeaways
Victoza (liraglutide) is a GLP-1 medication for type 2 diabetes, but it can also help with weight loss.
Liraglutide can support weight loss by reducing appetite and slowing stomach emptying, which may help you feel full for longer.
Victoza is a daily injection that may promote weight loss, but it’s not a quick fix — and it may not be the best GLP-1 for weight loss, depending on your situation.
Here's what we'll cover
Here's what we'll cover
Here's what we'll cover
Key takeaways
Victoza (liraglutide) is a GLP-1 medication for type 2 diabetes, but it can also help with weight loss.
Liraglutide can support weight loss by reducing appetite and slowing stomach emptying, which may help you feel full for longer.
Victoza is a daily injection that may promote weight loss, but it’s not a quick fix — and it may not be the best GLP-1 for weight loss, depending on your situation.
By now, you've probably heard that Ozempic, a weekly injection for type 2 diabetes, can cause significant weight loss. But is that also true of other injectable diabetes treatments? For instance, can you take Victoza for weight loss? Maybe.
Victoza can cause weight loss, but taking it for that purpose is considered off-label since it's only approved by the United States Food and Drug Administration (FDA) to manage diabetes, not for weight management (its active ingredient, liraglutide, is available under another brand name, Saxenda, which is approved for weight loss).
Keep reading for everything you need to know about Victoza for weight loss, including how it works, how it compares to other weight loss medications, side effects, and more.
Does taking Victoza help with weight loss?
Taking Victoza can help with weight loss, but results vary.
Victoza isn't technically a weight loss drug, but its active ingredient, liraglutide, is effective for weight loss and long-term weight management. In fact, liraglutide is sold under a different brand name, Saxenda, as an FDA-approved weight loss drug.
Victoza and Saxenda contain the same active ingredient but have different maximum doses — Victoza is prescribed up to 1.8 mg daily for managing diabetes, while Saxenda is used for weight management at a higher dose of up to 3 mg daily. Higher doses of liraglutide are generally more effective than lower doses for losing weight.
Like semaglutide, the active ingredient in Ozempic and Wegovy, liraglutide suppresses appetite and slows stomach emptying after meals. These effects can support weight loss efforts.
Here's what the science shows:
In a clinical trial of adults with type 2 diabetes already taking metformin, people who added liraglutide 1.2–1.8 mg lost around 6 pounds after six months, compared with 3 pounds on placebo, on average.
In one study, more people in the liraglutide group experienced meaningful weight loss (at least 5% or 10%) than the placebo group. After a little over a year, the participants lost an average of 14 pounds (6% of their body weight) on liraglutide 3 mg, 11 pounds (4.7%) on liraglutide 1.8 mg, and 5 pounds (2%) with placebo.
Clinical trials found that liraglutide (1.8–3 mg) effectively induced and sustained weight loss in people with obesity, including participants who also had weight-related conditions (e.g., high blood pressure or obstructive sleep apnea).
Though initially intended and approved to treat type 2 diabetes, research shows that Victoza can also help with weight loss. But results may be modest and weight loss tends to be greater at higher doses.
What is Victoza, exactly?
Victoza (liraglutide) is a prescription-only daily injection that’s FDA-approved to:
Control blood sugar levels in adults and children (age 10+) with type 2 diabetes, along with diet and exercise
Lower the risk of major cardiovascular problems like heart attack or stroke in adults with both type 2 diabetes and heart disease
Victoza belongs to a class of medications called glucagon-like peptide-1 receptor agonists, aka GLP-1s. These medications mimic the GLP-1 hormone, which is naturally released by the body while eating. This triggers the release of insulin, which lowers blood sugar levels, reduces appetite, and slows down digestion. Other GLP-1 medications include Ozempic (semaglutide), Wegovy (semaglutide), and Saxenda.
How does Victoza compare to other weight loss medications?
First, it's important to remember that Victoza is not technically a weight loss medication. It's a diabetes treatment that can cause weight loss.
That said, there are a number of FDA-approved weight loss medications on the market, including one that contains the same active ingredient as Victoza.
Other injectable weight loss medications include:
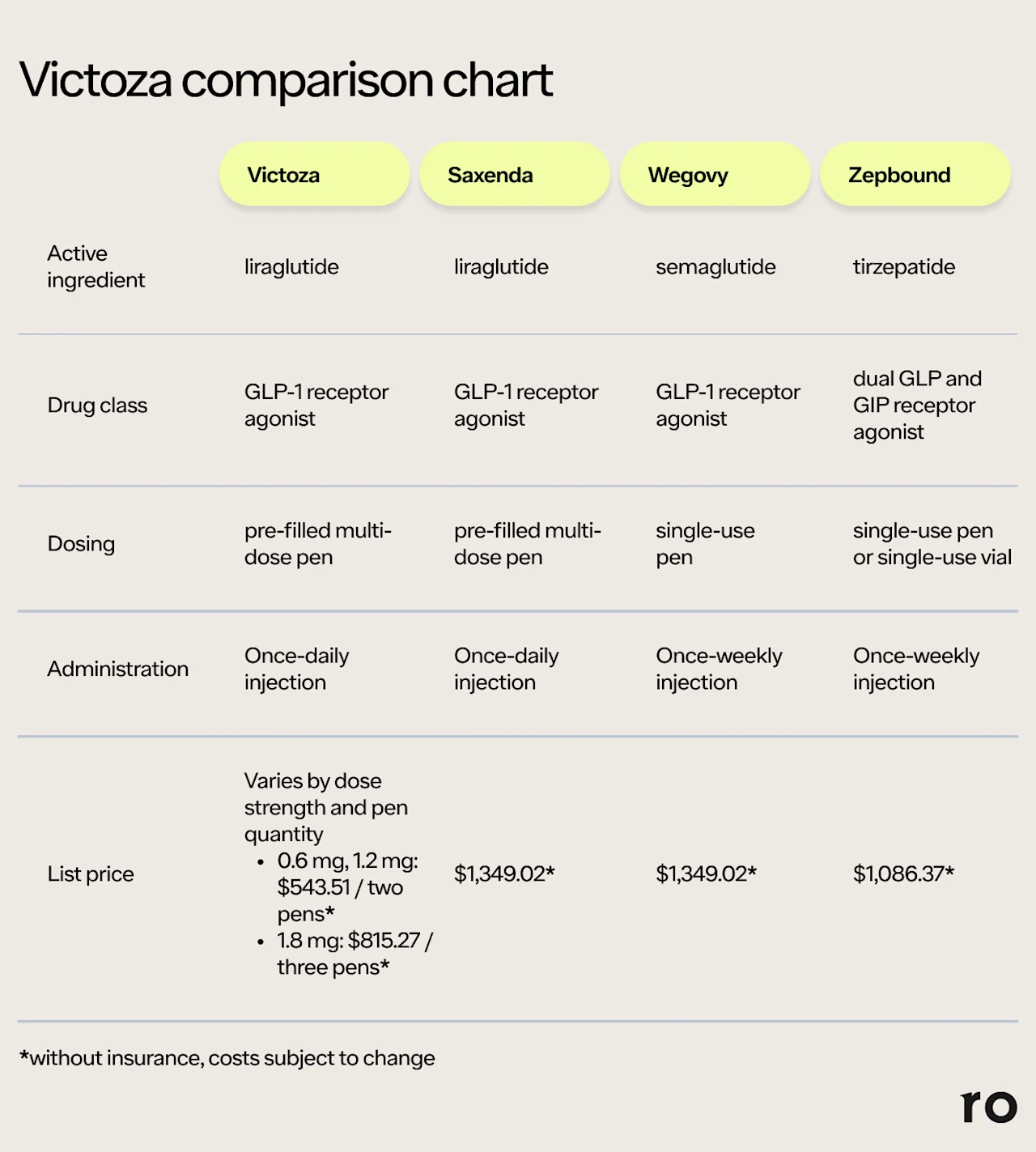
Saxenda: The GLP-1 liraglutide is also sold under the brand name Saxenda, which is a weight loss drug. It is FDA-approved for weight loss and weight management in people with obesity or overweight plus a weight-related condition (e.g., high blood pressure). Like Victoza, Saxenda is a once-daily injection — but Saxenda dosage goes up to 3 mg daily, whereas Victoza is only prescribed up to 1.8 mg daily.
Wegovy: Wegovy contains the GLP-1 receptor agonist semaglutide, the same active ingredient in Ozempic. Like Saxenda, Wegovy is an FDA-approved weight loss drug for people with overweight or obesity, among other uses. Unlike Victoza or Saxenda, Wegovy only needs to be injected once weekly. The maximum dose is 2.4 mg per week.
Zepbound: This once-weekly weight loss injection contains tirzepatide, a dual GLP/GIP receptor agonist. Zepbound's dual-action mechanism may explain why it often leads to more weight loss than Wegovy. Zepbound’s maximum dose for weight loss is 15 mg per week. In addition to weight management, the drug is also approved to treat obstructive sleep apnea in people with obesity.
Here's a quick comparison of the ingredients, dosing, and injection costs of Victoza, Saxenda, Wegovy, and Zepbound.
How much weight will I lose with Victoza?
There’s no guarantee of a specific amount of weight loss with Victoza. But some amount of weight loss on the drug is likely, especially for people who commit to lifestyle changes like a healthy (often calorie-restricted) diet and regular exercise.
Since it’s designed for type 2 diabetes, most studies of Victoza have focused on blood sugar control. Some also measured changes in body weight, giving us a sense of how much weight you may lose on Victoza:
Clinical trials found that people lost about 11–16 pounds after 20 weeks of daily liraglutide injections. However, participants’ doses went up to 3 mg — the maximum dose of Saxenda, not Victoza. (The maximum dose of Victoza is 1.8 mg.)
In a 56-week trial of people with type 2 diabetes, those who took 1.8 mg of liraglutide daily lost about 5 kg (11 pounds).
Another study found that combining liraglutide with exercise reduced body fat more than the drug or exercise alone. Participants who took 3 mg liraglutide per day and participated in vigorous exercise an average of 2.4 times per week decreased their body fat mass by 3.9% after one year.
The key takeaways here are that higher doses and regular exercise can increase how much weight you lose with Victoza.
While weight loss can be modest with Victoza, it can still be a good option compared to other diabetes medications that tend to cause weight gain, such as insulin and certain diabetes pills. For example, sulfonylureas such as glimepiride and glipizide can cause an average gain of nearly 9 pounds in the first year.
Since Saxenda is the same medication as Victoza, but approved for weight loss and available at higher doses, it might be a more beneficial choice if your primary goal is weight loss. Consult with your healthcare provider for personalized medical advice on the best GLP-1 medication for your specific needs.
Side effects of Victoza
The most commonly reported Victoza side effects include:
Nausea
Diarrhea
Vomiting
Upset stomach or indigestion
Constipation
Rare but serious side effects may include:
Pancreatitis (inflammation of the pancreas)
Gallbladder problems
Kidney problems or kidney failure
Dangerously low blood sugar (hypoglycemia), especially if also using insulin or certain diabetes pills (sulfonylureas)
Allergic reactions that may include swelling of the face, mouth, and throat, shortness of breath, rash, or fainting
If you experience severe abdominal pain, uncontrollable vomiting, or signs of an allergic reaction while on Victoza, stop taking it immediately and seek medical attention. Stay in contact with your healthcare provider about any concerns or adverse reactions that occur after starting Victoza.
How to take Victoza
Victoza comes in a pre-filled injection pen that you inject under your skin once a day, at the same time each day. The best time to take Victoza for weight loss (or any other reason) is the time you’re most likely to remember. The multi-dose pen delivers doses of 0.6 mg, 1.2 mg, or 1.8 mg. So, how many doses you get from one pen depends on the dose strength you’re prescribed.
Injecting Victoza every day might sound intimidating, but it is a straightforward process. Here are the steps, according to instructions from the drug’s manufacturer:
1. Gather your supplies
This includes:
Victoza pen (double-check to make sure it is your pen and the liquid inside is clear and colorless)
A new, sterile needle for the injection
An alcohol swab to clean the injection site
Gauze pad or cotton ball
A sharps disposal container
2. Wash your hands
Wash your hands with soap and water. Dry them with a clean towel or disposable paper towel. This step helps reduce the risk of getting bacteria in the injection site.
3. Attach the new needle
To inject Victoza, you’ll need pen needles that are sold or dispensed separately. (For example, NovoFine 32G pen needles — ask your prescriber or pharmacist for more details). The needles are individually packaged and covered by an inner and outer cap.
To attach a new needle:
Push the entire cap (containing the needle) onto the pen
Screw to tighten
Remove the outer needle cap and place it to the side
Remove the inner needle cap and discard it
4. Select your prescribed dose
If the pen is new, turn the dose selector “on” by twisting until the flow line (—) lines up with the pointer. Then, turn the dose selector until your prescribed dose is aligned with the pointer.
5. Prepare the injection site
Victoza can be injected into three different areas on the body:
Stomach
Thigh
Upper arm
Use a different injection site than the previous dose to avoid lipohypertrophy, a fatty lump that can develop under the skin after repeated injections in the same spot.
Once you’ve chosen your injection site, wipe it with an alcohol swab. Allow the skin to dry before injecting your dose.
6. Inject the medication
Hold the base of the pen flat against your skin, at a 90-degree angle. Be careful to avoid veins or muscles.
Press the needle into your skin using the technique demonstrated or described by your prescriber.
Press and hold the dose button for at least six seconds, until “0 mg” lines up with the pointer. At that point, lift the pen straight up to withdraw the needle.
If there is any blood, lightly press (don’t rub!) the gauze or cotton ball onto the injection site.
7. Safely dispose of the needle or pen
Now that the day’s Victoza injection is done, put the outer needle cap back over the needle and unscrew the needle from the pen.
Place the needle directly into an FDA-cleared sharps container or a heavy-duty plastic container with a lid (such as a laundry detergent bottle or a dishwashing pods container). Do not place used needles in your household trash.
If this was the last dose in your Victoza pen, you can also put the pen in the sharps container. If the pen contains more doses, replace the cap and store it in the refrigerator until your next dose, or you can leave it out at room temperature (59°F to 86°F) for up to 30 days.
Heads up: Victoza pens should be discarded after 30 days or when the medication is finished, whichever comes first.
Bottom line
Victoza is a type 2 diabetes treatment that often also causes weight loss. Here’s the recap:
Victoza is a GLP-1 medication approved for type 2 diabetes that can result in weight loss, according to clinical trials. It is not FDA-approved for weight loss, but it can be a good treatment option for people with type 2 diabetes who are also interested in losing weight. It may also be prescribed off-label for weight loss in people without diabetes.
Saxenda contains the same medication as Victoza (liraglutide) and is approved for weight loss. It’s prescribed in higher doses, so it tends to be more effective for weight loss.
There are several alternatives to Victoza for weight loss. Other injectable medications specifically prescribed for weight loss include Saxenda, Wegovy, and Zepbound.
GLP-1s like Victoza are not a silver bullet for obesity. Weight loss medications are intended to be used along with diet and exercise changes that promote weight loss.
Victoza isn’t right for everyone. A healthcare provider familiar with your other medications, medical history, and health and weight loss goals can help guide you toward the most suitable medications for your needs.
Frequently asked questions (FAQs)
What’s the difference between Victoza and Saxenda?
Victoza and Saxenda contain the same active ingredient, but they’re FDA-approved for different uses. Victoza is prescribed to help treat type 2 diabetes and lower cardiovascular risk, whereas Saxenda is prescribed in higher doses to help with weight loss.
Are there any alternatives to Victoza?
Yes. If you’re looking for an alternative to Victoza for weight loss, ask your prescriber about Saxenda, Wegovy, or Zepbound. Victoza alternatives for diabetes management include Ozempic, Rybelsus, and Mounjaro.
How do I get a prescription for Victoza?
How does Victoza cause weight loss?
Research shows that Victoza can cause weight loss by decreasing appetite and slowing down your digestion.
DISCLAIMER
If you have any medical questions or concerns, please talk to your healthcare provider. The articles on Health Guide are underpinned by peer-reviewed research and information drawn from medical societies and governmental agencies. However, they are not a substitute for professional medical advice, diagnosis, or treatment.
Ozempic Important Safety Information: Read more about serious warnings and safety info.
Saxenda Important Safety Information: Read more about serious warnings and safety info.
Wegovy Important Safety Information: Read more about serious warnings and safety info.
GLP-1 Important Safety Information: Read more about serious warnings and safety info.
Zepbound Important Safety Information: Read more about serious warnings and safety info.
References
Collins, L. & Costello, R. A. (2024). Glucagon-like peptide-1 receptor agonists. StatPearls. Retrieved on Oct. 15, 2025 from https://www.ncbi.nlm.nih.gov/books/NBK551568/
DailyMed. (2025). Liraglutide injection. Retrieved from https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2100ec49-57b2-4330-ab5f-057ce9b7e4d0
Davies, M. J., Bergenstal, R., Bode, B., et al. (2015). Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA, 314(7), 687–699. doi: 10.1001/jama.2015.9676. Retrieved from https://jamanetwork.com/journals/jama/fullarticle/2428956
Eli Lilly. (2025). How much should I expect to pay for Zepbound?. Retrieved on Oct. 15, 2025 from https://pricinginfo.lilly.com/zepbound
Hirsch, L. J. & Strauss, K. W. (2019). The injection technique factor: what you don't know or teach can make a difference. Clinical Diabetes : A Publication of the American Diabetes Association, 37(3), 227–233. doi: 10.2337/cd18-0076. Retrieved from https://pmc.ncbi.nlm.nih.gov/articles/PMC6640874/
le Roux, C. W., Hankosky, E. R., Wang, D., et al. (2023). Tirzepatide 10 and 15 mg compared with semaglutide 2.4 mg for the treatment of obesity: An indirect treatment comparison. Diabetes, Obesity & Metabolism, 25(9), 2626–2633. doi: 10.1111/dom.15148. Retrieved from https://pubmed.ncbi.nlm.nih.gov/37344384/
Jackson, S. H., Martin, T. S., Jones, J. D., et al. (2010). Liraglutide (victoza): the first once-daily incretin mimetic injection for type-2 diabetes. P & T : A Peer-Reviewed Journal for Formulary Management, 35(9), 498–529. Retrieved from https://pmc.ncbi.nlm.nih.gov/articles/PMC2957743/
Lundgren, J. R., Janus, C., Jensen, S. B. K., et al. (2021). Healthy weight loss maintenance with exercise, liraglutide, or both combined. The New England Journal of Medicine, 384(18), 1719–1730. doi: 10.1056/NEJMoa2028198. Retrieved from https://www.nejm.org/doi/10.1056/NEJMoa2028198
Mehta, A., Marso, S. P., & Neeland, I. J. (2017). Liraglutide for weight management: a critical review of the evidence. Obesity Science & Practice, 3(1), 3–14. doi: 10.1002/osp4.84. Retrieved from https://pmc.ncbi.nlm.nih.gov/articles/PMC5358074/
Novo Nordisk-a. (2025). Victoza 12 ® (liraglutide) injection 1.2 mg, 1.8 mg Instructions For Use. Retrieved from https://www.novo-pi.com/victoza.pdf#IFU
Novo Nordisk-b. (2025). Victoza Pricing. Retrieved on Oct. 15, 2025 from https://www.novopricing.com/victoza.html
NovoCare-a. (2025). What is the list price for Saxenda and will it impact me?. Novo Nordisk. Retrieved on Oct. 15, 2025 from https://www.novocare.com/obesity/products/saxenda/explaining-list-price.html
NovoCare-b. (2025). What is the list price for Wegovy and will it impact me?. Novo Nordisk. Retrieved on Oct. 15, 2025 from https://www.novocare.com/obesity/products/wegovy/let-us-help/explaining-list-price.html
Pi-Sunyer, X., Astrup, A., Fujioka, K., et al. (2015). A randomized, controlled trial of 3.0 mg of liraglutide in weight management. The New England Journal of Medicine, 373(1), 11–22. doi: 10.1056/NEJMoa1411892. Retrieved from https://www.nejm.org/doi/full/10.1056/NEJMoa1411892
U.S. Food & Drug Administration (FDA). (n.d.). Do’s and Don’ts: Safe disposal of needles and other sharps used at home, at work, or while traveling. Retrieved on Oct.15, 2025 from https://www.fda.gov/media/82389/download
U.S. Food and Drug Administration (FDA). (2025). SAXENDA (liraglutide) injection, for subcutaneous use. Retrieved on Oct. 15, 2025 from
https://www.accessdata.fda.gov/drugsatfda_docs/label/2025/206321s020lbl.pdf
U.S. Food and Drug Administration (FDA). (2024). VICTOZA® (liraglutide) injection, for subcutaneous use. Retrieved on Oct. 15, 2025 from https://www.accessdata.fda.gov/drugsatfda_docs/label/2025/022341s044lbl.pdf
Verhaegen, A. A. & Van Gaal, L. F. (2019). Drugs that affect body weight, body fat distribution, and metabolism. Endotext. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK537590/