Key takeaways
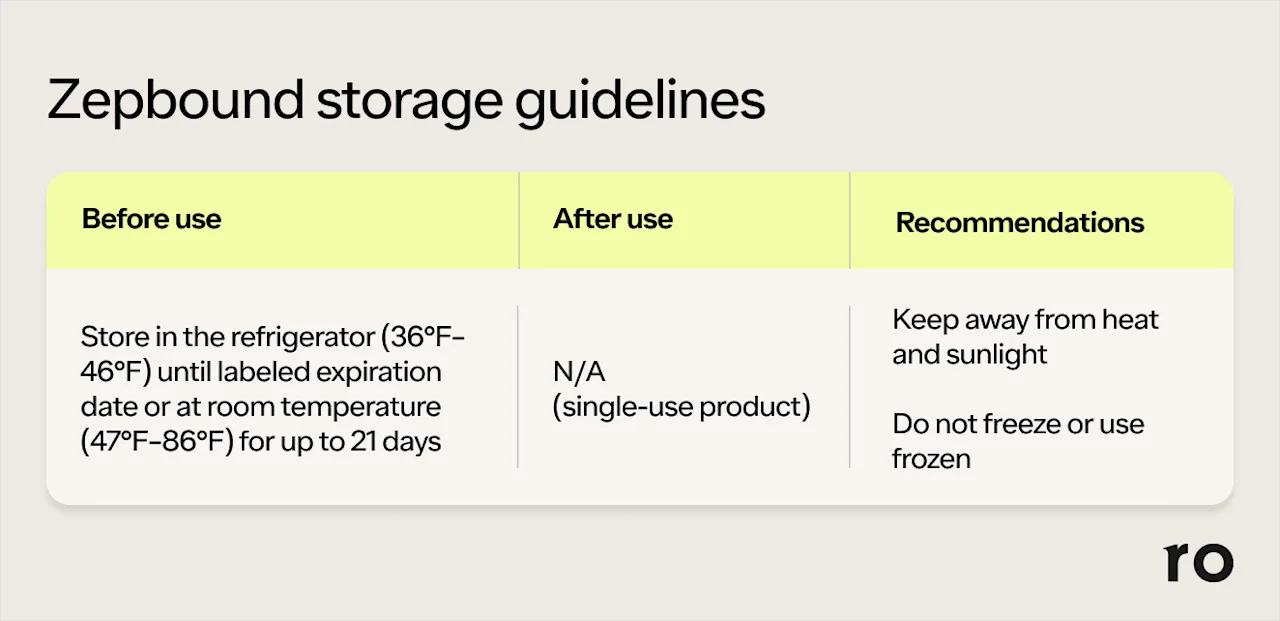
Before use, Zepbound should be refrigerated at 36°F–46°F(2°C–8°C) in its original packaging.
Unopened Zepbound can be kept at room temperature — up to 86°F (30°C) — for a maximum of 21 days.
Avoid using expired Zepbound, as its safety and effectiveness are not guaranteed.
Here's what we'll cover
Key takeaways
Before use, Zepbound should be refrigerated at 36°F–46°F(2°C–8°C) in its original packaging.
Unopened Zepbound can be kept at room temperature — up to 86°F (30°C) — for a maximum of 21 days.
Avoid using expired Zepbound, as its safety and effectiveness are not guaranteed.
If you’re considering or starting a Zepbound prescription for weight loss, you probably have questions about how and where to store the prefilled pens or single-dose vials. You might be wondering, “Does Zepbound need to be refrigerated?” The answer is yes, it should be kept in the refrigerator before use. Of course, this may spark even more inquiries like, “How long can Zepbound be out of the fridge before it goes bad?” or “How do I travel with Zepbound?”
Keep reading for everything you need to know about how to store Zepbound at home or on the go.
Does Zepbound need to be refrigerated?
Yes, you need to store Zepbound in the refrigerator, though it can be left unrefrigerated at room temperature for up to 21 days if necessary. This weight loss medication should be kept between 36°F and 46°F (2°C and 8°C) in its original packaging to protect it from heat and light exposure. Under no circumstances should you freeze Zepbound.
Not all refrigerators cool evenly, so keeping Zepbound on an open shelf toward the middle of the fridge can be a good idea. Don’t store Zepbound in direct contact with cooling elements, where it could develop ice crystals. Avoid keeping Zepbound in the refrigerator door, where temperatures fluctuate most.
Keeping Zepbound in the fridge means the liquid solution in your Zepbound pen or vial is quite cold. You shouldn’t use heat to warm the drug before injecting it, but you can take Zepbound out of the fridge and let it warm to room temperature prior to injecting if that makes administration more comfortable for you.
Friendly reminder: Zepbound pens and vials are all single-use, so you don’t need to return them to the refrigerator after use. Dispose of used injection supplies like pens, needles, and syringes in US Food and Drug Administration (FDA)-cleared sharps containers.
How long does Zepbound last in the fridge?
Zepbound can last months or even years when refrigerated at the proper temperatures, unopened, and within its original container. Exactly how long Zepbound lasts in the fridge depends on its expiration date, which can be found on the drug label.
Eli Lilly, the drug company that manufactures Zepbound, has given Zepbound injections an expiration date of two years (24 months) from the date of manufacture if the drug is kept at 36°F–46°F.
It’s important to remember that expiration dates are two years from when the drug is made, not dispensed. Always check the expiration dates on your Zepbound pens and vials to determine how long they will last in the fridge.
How long can Zepbound be left out of the fridge?
Zepbound can be stored at room temperature, meaning unrefrigerated, for up to three weeks (21 days), but should be protected from light and never exposed to temperatures above 86°F. These are the guidelines on the drug labels for both Zepbound pens and Zepbound vials.
Once stored at room temperature, Zepbound should not be re-refrigerated. Use it within 21 days of taking it out of the fridge — and if you don’t use it before then, safely discard it in a sharps container.
What happens if Zepbound is not refrigerated?
Leaving Zepbound unrefrigerated for more than the FDA-allotted 21 days may cause the active ingredient (tirzepatide) to degrade, compromising its safety and effectiveness.
If you’ve been prescribed Zepbound, you know that this is not a cheap medication. Follow Zepbound storage guidelines to make sure you are spending your money, time, and effort on a drug that is still safe and effective.
Does Zepbound expire?
Yes, Zepbound can expire. Typically, the drug’s expiration date is two years (24 months) after its manufacture date, as long as it's stored properly in the refrigerator (36°F–46°F). The expiration dating period for Zepbound is set by data from Eli Lilly, the drug manufacturer, and approved by the FDA.
Medication expiration dates are determined based on the manufacturer’s information about the stability of the drug’s ingredients. This is the manufacturer’s guarantee that storing the drug properly will keep it safe and effective until its expiration date.
Before using Zepbound, check the expiration date and make sure the liquid is clear to slightly yellow. Don’t use it if it is expired or cloudy or has particles.
What happens if you use expired Zepbound?
There is not enough data to know how safe and effective it is to use expired Zepbound. Expired Zepbound may be safe until a certain point — and it may still cause weight loss — but there is no guarantee. To stay safe, don’t use expired Zepbound pens or vials. Instead, talk with your pharmacist or healthcare provider about getting a new prescription.
How to travel with Zepbound
To stick to your dosage schedule, you must bring Zepbound with you if you’ll be traveling when your next weekly dose is due. The good news is that while traveling with Zepbound takes planning, it’s very straightforward.
First things first: Keep Zepbound in its original packaging. Beyond that, the main thing to consider is temperature. Zepbound pens and vials can remain unrefrigerated at temperatures between 47°F and 86°F for up to three weeks.
When traveling by plane, keep Zepbound in its labeled, original packaging. This ensures it remains protected from light and in compliance with airport regulations. Store Zepbound in your carry-on luggage so that it remains with you in temperature-controlled passenger areas. If you are traveling for more than three weeks or to a hot climate where temperatures may exceed 86°F, stash your Zepbound in an insulated bag with gel ice packs inside your carry-on. Medically necessary gel ice packs are allowed through security checkpoints in US airports — just alert the Transportation Security Administration (TSA) officer when you go through security. Check the airport website for regulations at international destinations.
When traveling by car or train, just like when flying, you can pack Zepbound in an insulated bag with gel ice packs to keep it cool. This is especially important when traveling in summer. Don’t pack it directly against ice, which could freeze the medication, and don’t leave it in the car in cold or freezing weather. Whether you decide to keep your meds in a cooler or at room temperature (up to 86°F) while driving, it’s important to keep Zepbound with you in the car. Meaning, do not put it in the trunk or anywhere it could be exposed to direct sunlight and heat.
All that said, don’t hesitate to consult your pharmacist or healthcare provider if you have trip-specific questions about traveling with Zepbound.
How to dispose of Zepbound
Zepbound comes in two forms: prefilled pens and vials that require a separate needle and syringe. None of these materials should be thrown away in household trash.
After each Zepbound injection, dispose of the supplies (pen, vial, needle, and/or syringe) in an FDA-approved sharps disposal container. If you’re unsure where to get an approved sharps disposal container, talk to your healthcare provider or pharmacist. You might also be able to buy one online.
If you don’t have a sharps container at home or while traveling, use a heavy-duty plastic container with a tight-fitting, puncture-resistant lid. A laundry detergent or fabric softener container may work well. Just make sure your sharps disposal container complies with any state or town guidelines when you are ready to dispose of the container later.
Bottom line
Whether in a pen or vial, Zepbound should be stored in its original packaging to protect the medication from light. It is best to store Zepbound in a refrigerator, though the medication can be stored at room temperature for up to three weeks.
Here’s a recap of the guidelines for safe Zepbound storage:
Ideally, you should store Zepbound in the fridge. Unopened Zepbound should be refrigerated and stored at 36°F–46°F until used or expired. Never freeze it.
If necessary, you can store Zepbound at room temperature. Unopened Zepbound can be stored at room temperature (up to 86°F) for up to three weeks. Never put it back into the refrigerator after storing it at room temperature.
Keep Zepbound in its original packaging. The most important things to remember when traveling with Zepbound are to keep it in its original container and to store it at consistent temperatures between 36°F and 86°F. Travels that last more than three weeks require storage at consistent temperatures between 36°F and 46°F.
Don’t use expired Zepbound. For best results, don’t use expired Zepbound. Store it properly — and if it looks cloudy or has been frozen, or exposed to heat, dispose of it in an FDA-approved sharps container.
Frequently asked questions (FAQs)
What happens if I don’t refrigerate Zepbound?
You can safely leave Zepbound out of the refrigerator at room temperature (up to 86°F) for a maximum of 21 days. Storing it at room temperature for more than three weeks may impact the safety and effectiveness of this medication.
Should I let Zepbound come to room temperature?
You don’t need to let Zepound come to room temperature before injecting it, but you can. Research suggests that injecting cold liquid solutions may cause more pain than room-temperature solutions, though experiences can vary.
How long does a vial of Zepbound last?
When stored at 36°F–46°F in its original packaging and carton, an unopened vial of Zepbound lasts until its expiration date. In general, Zepbound expires two years (24 months) after its manufacture date. The expiration date is printed on the vial and the outer carton.
Should you inject Zepbound in the morning or at night?
You can inject Zepbound at any time of day. The most important thing is to be consistent about injecting Zepbound on the same day each week. Pick a day and time of day that is convenient and easy to remember.
DISCLAIMER
If you have any medical questions or concerns, please talk to your healthcare provider. The articles on Health Guide are underpinned by peer-reviewed research and information drawn from medical societies and governmental agencies. However, they are not a substitute for professional medical advice, diagnosis, or treatment.
Zepbound Important Safety Information: Read more about serious warnings and safety info.
References
Arioua, A. & Shaw, D. (2024). Use of expired drugs: patients benefits versus industry interest. JMA Journal, 7(1), 1–4. doi: 10.31662/jmaj.2022-0209. Retrieved from https://pmc.ncbi.nlm.nih.gov/articles/PMC10834166
Bainbridge, L. C. (1991). Comparison of room temperature and body temperature local anaesthetic solutions. British Journal of Plastic Surgery, 44, 147–148. Retrieved from https://www.jprasurg.com/article/0007-1226(91)90050-T/pdf
Transportation Security Administration (TSA-a). (n.d.). Disabilities and medical conditions. Retrieved from https://www.tsa.gov/travel/tsa-cares/disabilities-and-medical-conditions
Transportation Security Administration (TSA-b). (n.d.). Gel Ice Packs. Retrieved from https://www.tsa.gov/travel/security-screening/whatcanibring/items/gel-ice-packs
Transportation Security Administration (TSA-c). (n.d.). I am traveling with medication, are there any requirements I should be aware of?. Retrieved from https://www.tsa.gov/travel/frequently-asked-questions/i-am-traveling-medication-are-there-any-requirements-i-should-be
U.S. Department of Agriculture (USDA). (2015). Refrigeration and Food Safety. Retrieved from https://www.fsis.usda.gov/food-safety/safe-food-handling-and-preparation/food-safety-basics/refrigeration
U.S. Food & Drug Administration (FDA-a). (2023). Approval letter, NDA 217806. Retrieved from https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2023/217806Orig1s000ltr.pdf
U.S. Food and Drug Administration (FDA-b). (2023). Best Way to Get Rid of Used Needles and Other Sharps. Retrieved from https://www.fda.gov/medical-devices/safely-using-sharps-needles-and-syringes-home-work-and-travel/best-way-get-rid-used-needles-and-other-sharps
U.S. Food & Drug Administration (FDA). (2025). Highlights of Prescribing Information: Zepbound (tirzepatide) injection, for subcutaneous use. Retrieved from https://www.accessdata.fda.gov/drugsatfda_docs/label/2025/217806Orig1s020lbl.pdf
U.S. Food & Drug Administration (FDA). (2024). Refrigerator Thermometers - Cold Facts about Food Safety. Retrieved from https://www.fda.gov/food/buy-store-serve-safe-food/refrigerator-thermometers-cold-facts-about-food-safety
U.S. Food & Drug Administration (FDA). (2021). Sharps Disposal Containers. Retrieved from https://www.fda.gov/medical-devices/safely-using-sharps-needles-and-syringes-home-work-and-travel/sharps-disposal-containers