Here's what we'll cover
Here's what we'll cover
Here's what we'll cover
When it comes to allergy medications, there are lots of choices even within the same drug class. Antihistamines are some of the most commonly used allergy drugs—and there are several to choose from. So how do you know which to choose? We are going to break down the similarities and differences between two popular antihistamines: Zyrtec vs. Xyzal.
What are seasonal allergies?
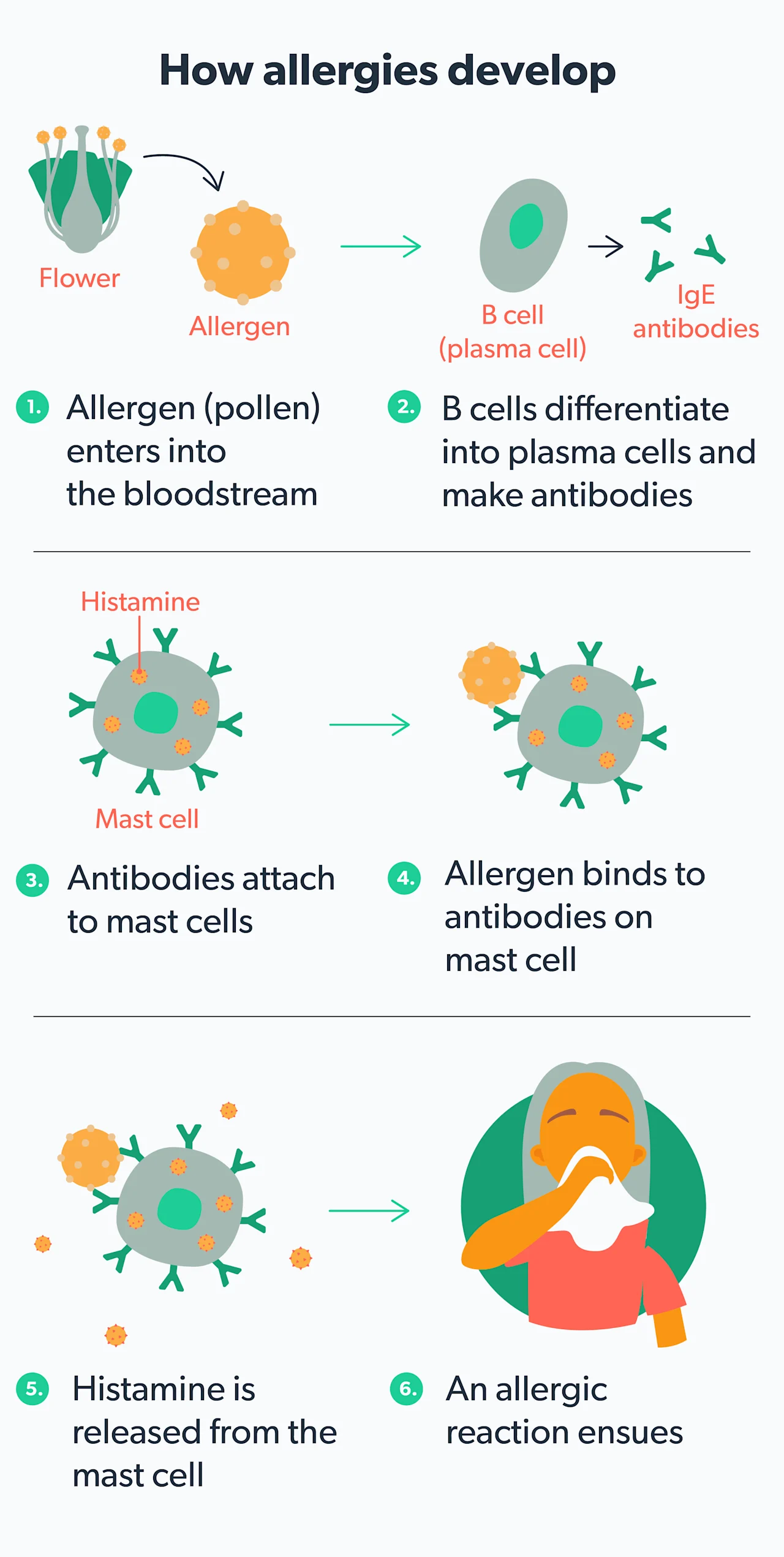
Seasonal allergies can be annoying, but they serve a specific purpose: to protect your body from "intruders" that may harm you. When your immune system becomes overly sensitive to a particular substance (called an allergen), it tries to defend you by fighting off the trigger with a release of inflammatory chemicals. Unfortunately, these chemicals also cause the symptoms of allergies.
People with seasonal allergies (also known as allergic rhinitis or hay fever) may only experience allergy symptoms like sneezing, runny nose, coughing, and nasal congestion at certain times of the year. For example, people who are allergic to certain tree pollens like oak, maple, or birch may experience allergy symptoms in the spring. In contrast, people who are sensitive to grass pollens and weed pollens may sneeze and cough through summer and fall. Some people also experience "seasonal" allergy symptoms year-round—these are called perennial allergies—usually to allergens like pet dander and dust mites (Akhouri, 2021).
Allergy treatments include avoiding the allergens (when possible), natural remedies, medications (like antihistamines, nasal steroids, decongestants, etc.), and—in some cases—immunotherapy with allergy shots or allergy drops.
What are antihistamines?
When your body encounters an allergen that you are sensitive to (like pollen), your immune system releases a chemical messenger called histamine. Histamine triggers the classic allergy symptoms of itching, runny nose, sneezing, allergic rhinitis/hay fever, etc. Antihistamines are allergy medications that prevent histamine from attaching to immune cells in your body, triggering your allergy symptoms. This class of drugs is often broken down into different generations (Farzam, 2021).
Allergy medications like diphenhydramine, the active ingredient in Benadryl, are considered first-generation antihistamines. These first-generation antihistamines work well for your allergies, but they can cause significant sedation (drowsiness).
Since most people can't afford to be sleepy throughout the entire allergy season, second-generation antihistamines, which are effective but with less chance of drowsiness, are often used to treat allergy symptoms. Some examples include Claritin (active ingredient loratadine), Allegra (active ingredient fexofenadine), and Zyrtec (active ingredient cetirizine) (Farzam, 2021).
What is Zyrtec?
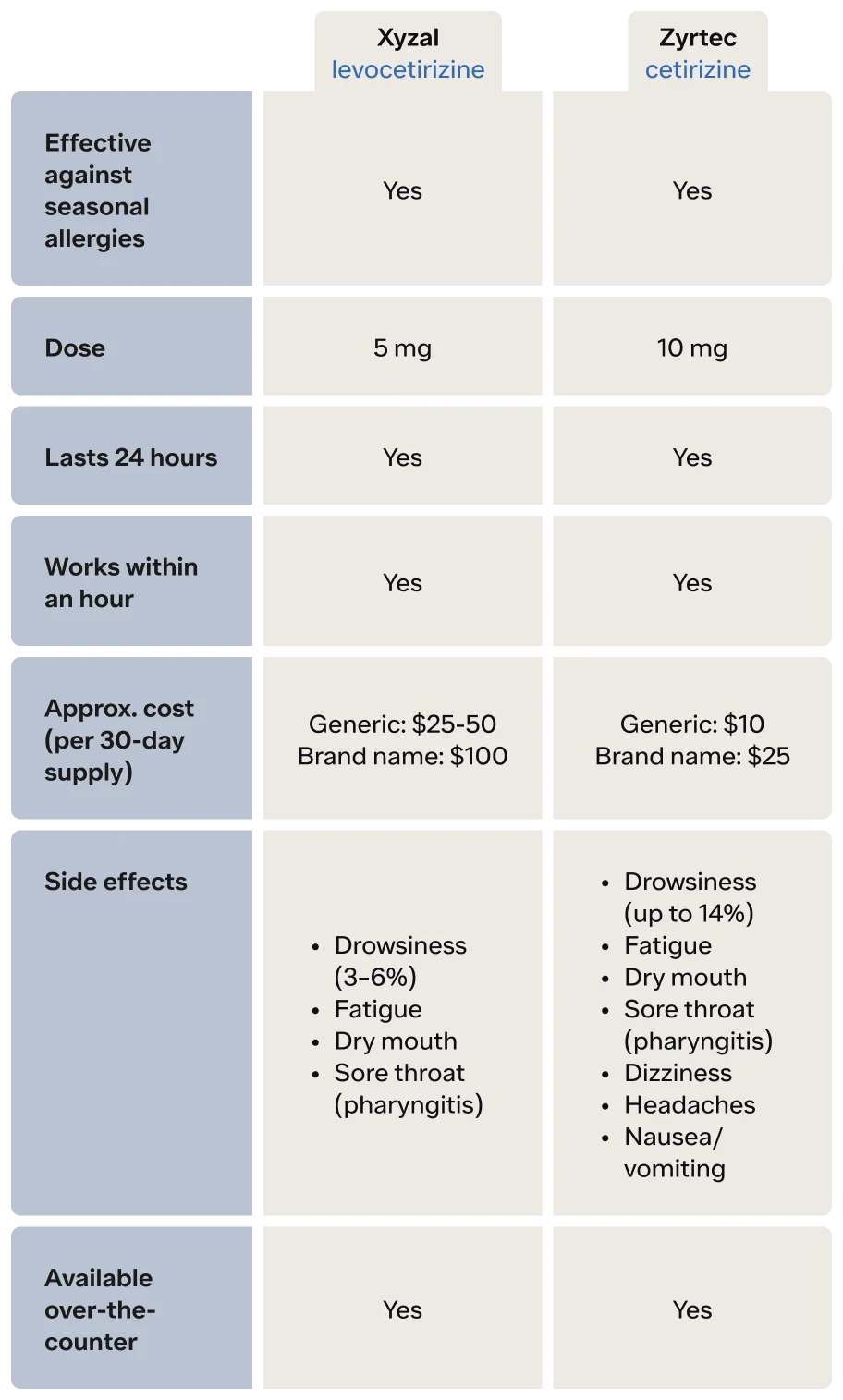
The active ingredient of Zyrtec is a second-generation antihistamine, cetirizine. This allergy medication generally does not cause as much sedation as first-generation antihistamines, but it does cause drowsiness in some people. In clinical trials, up to 14% of people reported some sleepiness. Zyrtec works to treat seasonal allergy symptoms like itchy, runny nose, itchy eyes, nasal congestion, sneezing, and hives in both adults and children as young as two years of age (UpToDate, n.d.-a).
Zyrtec begins working quickly—within the first hour after taking it—and can last 24 hours (Day, 2001). It is available over-the-counter (OTC), and forms of Zyrtec include tablets, syrup, dissolving tablets, and liquid gels.
What is Xyzal?
Xyzal is an even newer drug—a third-generation antihistamine. The generic name of Xyzal is levocetirizine. If you think that this allergy medication looks like the active ingredient of Zyrtec (cetirizine), you are correct. Xyzal is formulated with a specific form of cetirizine (UpToDate, n.d.-b)
Just like Zyrtec, Xyzal works to treat seasonal allergy symptoms like itchy, runny nose, itchy eyes, nasal congestion, sneezing, and hives in both adults and children as young as two years of age. Xyzal begins working within the first hour after taking it and can last 24 hours. It is over-the-counter, and forms of Xyzal include tablets and syrup.
Side effects
Even though Zyrtec and Xyzal are similar chemically, there are differences in their side effect profiles. Zyrtec side effects (FDA, 2002)
Drowsiness (up to 14%)
Fatigue
Dry mouth
Sore throat (pharyngitis)
Dizziness
Headaches
Nausea/vomiting
Xyzal side effects (FDA, 2008)
Drowsiness (3–6%)
Fatigue
Dry mouth
Sore throat (pharyngitis)
Zyrtec and Xyzal may cause drug interactions or other side effects, so it’s always important to get medical advice from your healthcare provider about all the pros and cons of any medications or supplements you take.
Differences and similarities of Xyzal vs. Zyrtec
When it comes to treating your allergies, several options are available. Over-the-counter antihistamines, especially the second and third-generation drugs, are some of the most popular. Talk to your healthcare provider if you have any questions regarding which one is right for you.
DISCLAIMER
If you have any medical questions or concerns, please talk to your healthcare provider. The articles on Health Guide are underpinned by peer-reviewed research and information drawn from medical societies and governmental agencies. However, they are not a substitute for professional medical advice, diagnosis, or treatment.
References
Akhouri, S. & House, S. A. (2021). Allergic rhinitis. [Updated Mar 31, 2021]. In: StatPearls [Internet]. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK538186/
Day, J., Ellis, A., & Rafeiro, E. (2004). Levocetirizine: A new selective H1 receptor antagonist for use in allergic disorders. Drugs of Today , 40 (5), 415. doi: 10.1358/dot.2004.40.5.850489. Retrieved from https://pubmed.ncbi.nlm.nih.gov/15319796/
Farzam, K., Sabir, S., & O'Rourke, M. C. (2021). Antihistamines. [Updated Jul 18, 2021]. In: StatPearls [Internet]. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK538188/
UptoDate. (n.d.-a). Cetirizine (systemic): drug information. Retrieved on Jul 1, 2021 from https://www.uptodate.com/contents/cetirizine-systemic-drug-information
UptoDate. (n.d.-a). Levocetirizine: drug information. Retrieved on Jul 1, 2021 from https://www.uptodate.com/contents/levocetirizine-drug-information
U.S. Food and Drug Administration (FDA). (2002). ZYRTEC® (cetirizine hydrochloride). Retrieved on Jul 1, 2021 from https://www.accessdata.fda.gov/drugsatfda_docs/label/2002/19835s15,%2020346s8lbl.pdf/
U.S. Food and Drug Administration (FDA). (2008). Xyzal (levocetirizine dihydrochloride). Retrieved on Jul 1, 2021 from https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/022064s009lbl.pdf