Key takeaways
Before opening, Ozempic should be refrigerated at 36°F–46°F. It stays good at these temperatures until the labeled expiration date.
After first use, the medication can be stored at room temperature (59°F–86°F) or in the refrigerator for up to 56 days.
When it comes to proper Ozempic storage, be sure to keep the medication away from extreme temperatures and direct light. Improper storage conditions could change the drug’s composition and, in turn, its effectiveness.
Here's what we'll cover
Key takeaways
Before opening, Ozempic should be refrigerated at 36°F–46°F. It stays good at these temperatures until the labeled expiration date.
After first use, the medication can be stored at room temperature (59°F–86°F) or in the refrigerator for up to 56 days.
When it comes to proper Ozempic storage, be sure to keep the medication away from extreme temperatures and direct light. Improper storage conditions could change the drug’s composition and, in turn, its effectiveness.
Before starting a new medication, you’re bound to have questions—and that’s no different when it comes to taking Ozempic (semaglutide). You might wonder how to store it between doses, especially if you’re on the go or planning a trip. Questions like, “does Ozempic need to be refrigerated?” or “what happens if it’s left out too long?” are important to understand for both safety and effectiveness.
In short, unopened Ozempic pens need to be refrigerated until first use or until their labeled expiration date; opened Ozempic pens can be stored at room temperature or in the fridge. But, again, that’s just the short of it. Let’s dive deeper into the topic of Ozempic storage so you can be confident that your medication stays effective and safe, whether you’re just living life at home or traveling the world.
Ozempic Important Safety Information: Read more about serious warnings and safety info.
Does Ozempic need to be refrigerated?
Yes, unopened Ozempic pens should be refrigerated until you're ready to use them for the first time. Keeping the medication between 36°F and 46°F helps ensure the ingredients do not degrade, which, in turn, helps preserve the drug and maintain its stability and purity until first use.
Avoid storing your pen(s) in the refrigerator door since it opens and closes throughout the day. This could potentially prevent the drug from staying cold enough to maintain its effectiveness. Similarly, keep Ozempic pens away from your refrigerator’s cooling element (usually located at the back or top of the fridge) because they could get too cold or freeze. If an Ozempic pen freezes, the medicine's chemical composition can change, potentially making it less effective. Novo Nordisk, the manufacturer of Ozempic, recommends discarding your Ozempic if it has frozen.
When unopened and stored properly in the fridge, Ozempic pens can last up until the expiration date printed on the box.
Now, does Ozempic need to be refrigerated after opening? It can be, but it doesn’t have to. Once opened, you can store your Ozempic pen at room temperature (59°F–86°F) or in the fridge (36°F–46°F) for up to 56 days.
Whether you store your opened Ozempic in the fridge or at room temperature, it’s best to keep it away from light and heat. To protect the medicine from light, you should replace the pen cap after each dose. Just as with unopened pens, the key to opened Ozempic storage is keeping the drug in an environment with a relatively stable temperature as exposure to extreme heat or cold can impact the medication’s potency.
To keep your medication working well, here are some simple Ozempic storage tips:
Avoid keeping Ozempic pens in the refrigerator door, where temperatures can fluctuate.
Don’t put Ozempic near the fridge's cooling elements, which are usually located in the back wall or ceiling, as these spots can get too cold.
Don’t freeze Ozempic; if it does freeze, don’t use it.
Keep the pen away from light and heat.
Place the cap on the Ozempic pen when you’re not using it.

Can you inject Ozempic cold?
As soon as you take Ozempic out of the refrigerator, you can use it. While injecting Ozempic cold is perfectly safe, you may experience stinging at the injection site. Keeping your Ozempic pen at room temperature after it's opened may make your injection more comfortable. Before using a new Ozempic pen for the first time, you have the option to take it out of the fridge ahead of time to allow it to reach room temperature. (But don’t put the drug in direct sunlight or use any other method to warm it up. Doing so could ruin the medication.)
What happens if Ozempic is left unrefrigerated?
I left my Ozempic out overnight—what should I do? The answer depends on whether or not the pen has been opened and used.
If your Ozempic pen was left out overnight and you have already used at least one dose of it, you can continue to use it. Ozempic pens can be left at room temperature (59°F–86°F) for up to 56 days after being used for the first time.
However, Ozempic should always be stored in the refrigerator until its first use. If you left your Ozempic pen out overnight before its first use, contact your pharmacist or healthcare provider before using it. They may recommend that you dispose of that pen and start a new one before your next weekly injection, especially if Ozempic was left, say, out in the car and was exposed to extreme temperatures. This is because a temperature change could impact how the medication works. If you have questions about whether your Ozempic is safe to use, you can also call the manufacturer at 1-800-727-6500.
No matter how you store the pen, the liquid inside it (i.e. the medication) should be clear and colorless before and throughout use. (You can see the color of the medication in the window area of the pen near the tip when the cap is off.) If the medicine appears discolored (or feels warm or frozen), you should contact your healthcare provider before using the pen.
It's always best to contact your healthcare provider if you have any doubts about your medication before taking it. If you do use your Ozempic pen without knowing if it was stored properly, the drug may not work as effectively. While you shouldn’t give a questionable pen a go without first consulting your provider, any signs that medicine isn’t working, such as high blood glucose, may also be a sign a pen is no longer effective.
How to dispose of Ozempic
Ozempic comes with Novofine pen needles. You’ll use a new one for each injection. After use, you should not toss the needles in the trash. Instead, dispose of Ozempic (or any medication with needles and syringes, for that matter) and pen needles in a sharps disposal container cleared by the US Food and Drug Administration (FDA).
To make this process easier, Novo Nordisk offers a free sharps disposal program. Once you complete a request form online, the company will send you a proper FDA-cleared disposal container with instructions on how to return it once full.
You may also be able to make your own by using a household container composed of heavy-duty plastic with a tight-fitting, puncture-resistant lid, such as a laundry detergent container. Just make sure the non-sharps alternative is in accordance with your state or town’s guidelines as is how you dispose of the container once full. According to the FDA, there may also be local drop-off sites for sharps, such as at doctors' offices, hospitals, pharmacies, health departments, and police or fire stations.
Does Ozempic expire?
Yes, Ozempic can expire. Typically, the drug's expiration date is 24 months from the date of manufacture, as long as it’s stored properly in the refrigerator (36°F–46°F). The expiration date is stamped or printed on the outer carton and on each pen after the text, “EXP.”
Opened Ozempic, on the other hand, only lasts up to 56 days after first use—that is, as long as it’s stored properly at room temperature (59°F–86°F) or in the fridge.
If you're taking Ozempic as prescribed and are careful to use up one pen before opening the next one, you should not have leftover medication. But, just in case, always note when you open your Ozempic pen, so you know to discard any unused medication after 56 days.
What happens if you use expired Ozempic?
It isn’t known for sure what happens after injecting expired Ozempic. This scenario hasn’t been specifically studied. A drug’s expiration date represents how long the manufacturer guarantees the medication will stay safe and effective, assuming it remains sealed and properly stored. Drug companies determine this date through stability testing, which looks at how factors like temperature, humidity, and time affect the drug.
After a drug’s expiration date has passed, all bets are off, meaning it may not work as intended. This is especially likely for temperature-sensitive injectable solutions that require refrigeration, like Ozempic. Expired medications could also break down or change in chemical composition, possibly leading to uncertain risks or side effects. If your Ozempic has expired or if the solution looks discolored or contains solid particles, it’s best to contact your pharmacy or prescriber to get a fresh supply.
How to store Ozempic while traveling
The specific storage method you opt for when traveling largely depends on whether or not your Ozempic is opened.
If you are traveling with unopened pens, consider using an insulated travel case, similar to the ones used for insulin pens, to keep your medication at the necessary cold temperature and, in turn, effective. You can store an opened Ozempic pen in an insulated container as well or at a stable room temperature (59°F–86°F)—i.e. just as you would at home.
When traveling by plane, make sure to keep Ozempic separate from the rest of your carry-on items when going through security. You may also want to travel with your prescription-labeled Ozempic carton. Also, it’s best to store Ozempic in a carry-on, not a checked bag, since luggage can be delayed or lost, and you can’t control the temperature of a plane’s cargo hold area.
When traveling by car, the same recommendations apply here as well: keep any unopened Ozempic pens in an insulated travel pack or cooler, taking care not to freeze them. If you're traveling with opened Ozempic pens, you don't need to refrigerate them, but you should keep them in a spot where they aren't exposed to high heat or extreme cold. Meaning, you don’t want to pack them away in the trunk or leave them inside a hot car that’s parked in direct sunlight.
Once you arrive at your destination, you should transfer your unopened pens to a refrigerator for the duration of your trip. As for opened pens, you can stash those as you would at home.
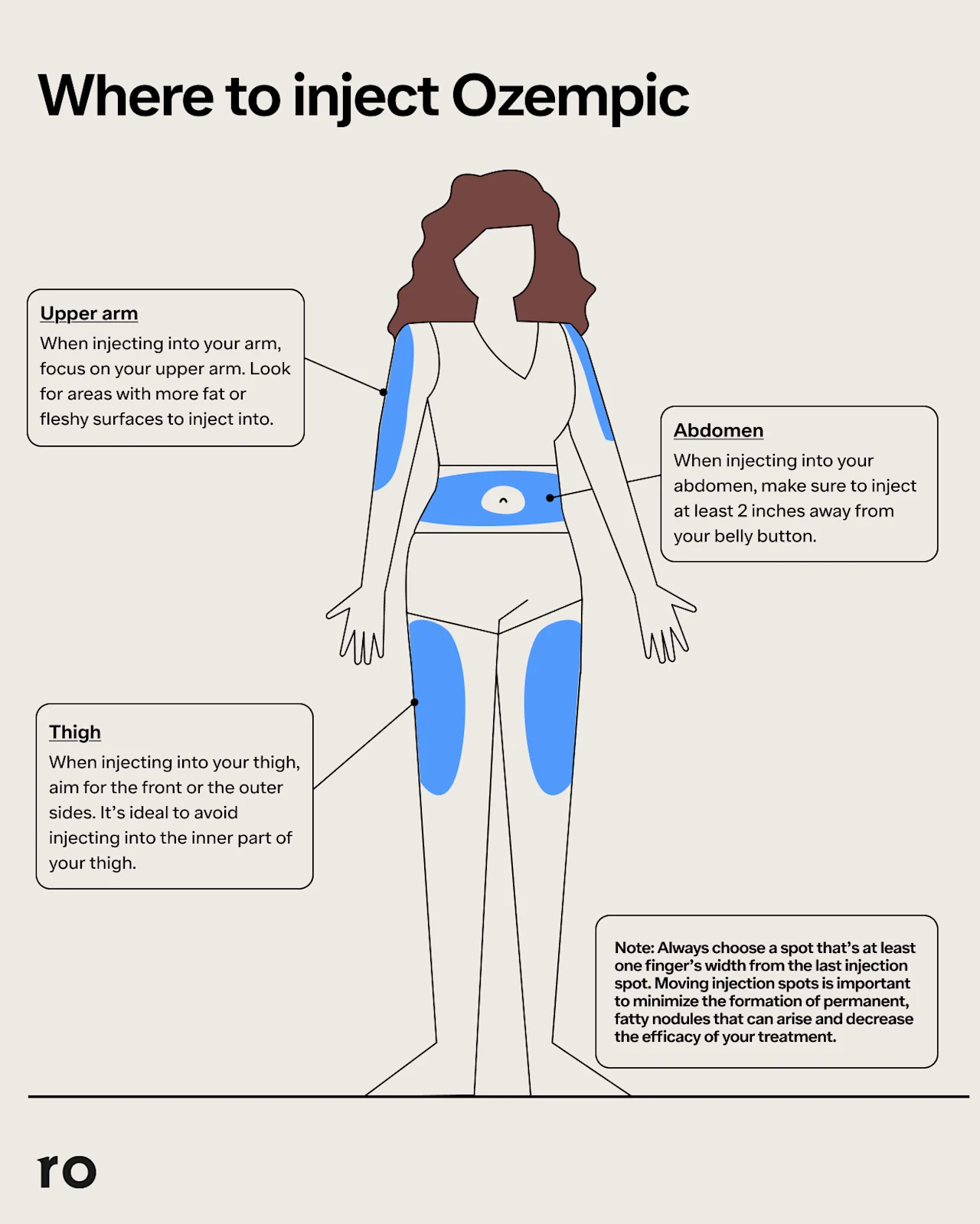
How to use an Ozempic pen
If you’re using your Ozempic pen for the first time, you can reach out to your doctor or pharmacist for tips or read through the manufacturer's instructions. Here are the steps to inject an Ozempic pen correctly:
Wash your hands with soap and water and remove the Ozempic pen cap.
Attach a new needle to your pen. (Use a new needle every time and do not share needles with anyone else.)
Clean the area of skin where you'll be injecting with an alcohol swab and allow the skin to fully air dry.
If it's your first time using Ozempic, check the flow by turning the selector dial until the dose counter shows the flow-check symbol, then press and hold the dose button until a drop appears at the needle tip. (You only need to do this the first time you use a pen.)
Next, use the dose selector dial to select your prescribed dose.
Insert the Ozempic needle into your skin at the chosen injection site (abdomen, thigh, or upper arm).
Press the dose button and hold it down until the 0 mg mark aligns with the dose indicator.
Keep the button pressed and the needle in place for six seconds to ensure the entire dose is delivered.
With your thumb still on the button, carefully withdraw the needle from your skin.
Remove the needle and dispose of it properly in a sharps container.
Replace the pen cap and store the pen in your fridge or at room temperature.
You can take Ozempic with or without food, and you should alternate injection sites each week. If you have any questions about taking Ozempic, reach out to your pharmacist or provider.
Bottom line: how to store Ozempic
The right way to store Ozempic comes down to whether the pen is sealed or opened. Unopened Ozempic pens must be stored in a refrigerator. Opened Ozempic pens can be stored at room temperature. Neither opened nor unopened Ozempic pens should be frozen or exposed to heat. Here are the guidelines to keep in mind:
Unused Ozempic pens should be stored in a refrigerator at 36°F–46°F. As soon as you bring your Ozempic pens home from the pharmacy or open the box if you receive the drug via home delivery, place them in a refrigerator. Avoid storing the pens in the fridge door, as the opening and closing could expose them to variable temperatures.
After first use, you can store your Ozempic pen at room temperature (59°F–86°F) or in the refrigerator. Just as you would with unopened pens, make sure to avoid extreme temperatures, as they can change the medication’s chemical composition, possibly making it less effective or posing unknown risks.
Opened Ozempic pens expire 56 days after first use. Always check the expiration date on your Ozempic pen and note the date you opened it. Unopened pens can last up until the labeled expiration date, as long as they’re stored properly.
If you’re unsure whether the medication was stored properly or you notice the liquid is discolored or feels warm or frozen, do not use it. Instead, contact your healthcare provider or pharmacist for guidance on next best steps.
Remember: If you have questions about Ozempic storage, talk to your pharmacist or healthcare provider. They will provide medical advice, review possible side effects, and help answer any questions you may have to ensure you get the best results from your prescription diabetes medication.
Frequently asked questions (FAQs)
How long can you leave Ozempic out for?
Unopened Ozempic pens should be stored in the refrigerator at 36°F–46°F until first use or the labeled expiration date. Once opened, it's safe to leave Ozempic pens out at room temperature (59°F–86°F) for up to 56 days—providing, of course, the medication is not exposed to any extreme temperatures and is stored properly.
What happens if Ozempic isn’t refrigerated?
If Ozempic is not opened and is not refrigerated, it could degrade the medication and make it less effective. Once Ozempic is opened, it can safely be stored at room temperature (59°F–86°F) for up to 56 days from first use.
How can you keep Ozempic cold while traveling?
Your best bet for keeping unopened Ozempic pens cold while traveling is by storing them in a cooler or insulated bag with gel ice packs. Avoid using ice cubes, as freezing could damage the medication. When flying, be sure to keep the medication in your carry-on bag, as the cargo hold can expose your medication to extreme temperatures. The same general advice applies when traveling via car: avoid extreme temperatures and keep your medication with you rather than, say, in the trunk. If your Ozempic pen is opened, you don’t need to worry about keeping it cold while traveling. That’s because, after first use, Ozempic can be kept at room temperature(59°F–86°F) for up to 56 days.
DISCLAIMER
If you have any medical questions or concerns, please talk to your healthcare provider. The articles on Health Guide are underpinned by peer-reviewed research and information drawn from medical societies and governmental agencies. However, they are not a substitute for professional medical advice, diagnosis, or treatment.
References
Jacob, J. J. (2023). Insulin Storage Guidance for Patients with Diabetes Using Insulin. Indian Journal of Endocrinology and Metabolism, 27(2), 93–95. doi: 10.4103/2230-8210.374161. Retrieved from https://journals.lww.com/indjem/fulltext/2023/03000/insulin_storage_guidance_for_patients_with.1.aspx
JAMA Network. (2016). Drugs past their expiration date. JAMA, 315, 5, 510-511. doi: 10.1001/jama.2016.0048. Retrieved from https://jamanetwork.com/journals/jama/fullarticle/2484679
National Library of Medicine. (2024). Semaglutide Injection. Retrieved from https://medlineplus.gov/druginfo/meds/a618008.html
Nauck, M. A., Quast, D. R., Wefers, J., et al. (2021). GLP-1 receptor agonists in the treatment of type 2 diabetes – state-of-the-art. Molecular Metabolism, 2021(46), 101102. doi: 10.1016/j.molmet.2020.101102. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8085572/
Novo Nordisk-a. (n.d.). Frequently asked questions about Ozempic® (semaglutide) injection. Retrieved from https://www.novomedlink.com/diabetes/products/treatments/ozempic/about/frequently-asked-questions.html
Novo Nordisk-b. (n.d.). How to safely dispose of used Novo Nordisk products. Retrieved from https://www.novocare.com/diabetes/resources/safe-disposal.html
Novo Nordisk. (2025). Ozempic Semaglutide Injection. Retrieved from https://www.novo-pi.com/ozempic.pdf
Novo Nordisk-c. (n.d.). Using your Ozempic pen. Retrieved from https://www.ozempic.com/how-to-take/ozempic-pen.html
Transportation Security Administration (TSA). (n.d.). Travel Tip: Traveling with Diabetes. Retrieved from https://www.tsa.gov/blog/2020/11/13/travel-tip-traveling-diabetes
U.S. Customs and Border Protection. (n.d.). Traveling with Medication. Retrieved from https://www.help.cbp.gov/s/article/Article-1444
U.S. Food and Drug Administration (FDA). (2023). Best Way to Get Rid of Used Needles and Other Sharps. Retrieved from https://www.fda.gov/medical-devices/safely-using-sharps-needles-and-syringes-home-work-and-travel/best-way-get-rid-used-needles-and-other-sharps
U.S. Food and Drug Administration (FDA). (n.d.). Disposal of Unused Medicines: What You Should Know. Retrieved from https://www.fda.gov/drugs/safe-disposal-medicines/disposal-unused-medicines-what-you-should-know
U.S. Food and Drug Administration (FDA). (2024). Don’t Be Tempted to Use Expired Medicines. Retrieved from https://www.fda.gov/drugs/special-features/dont-be-tempted-use-expired-medicines
U.S. Food and Drug Administration (FDA). (2025). Prescribing information: Ozempic (semaglutide) injection, for subcutaneous use. Retrieved from https://www.accessdata.fda.gov/drugsatfda_docs/label/2025/209637s025lbl.pdf
U.S. Food and Drug Administration (FDA). (2021). Sharps disposal containers. Retrieved from https://www.fda.gov/medical-devices/safely-using-sharps-needles-and-syringes-home-work-and-travel/sharps-disposal-containers
Usach, I., Martinez, R., Festini, T., & Peris, J. E. (2019). Subcutaneous Injection of Drugs: Literature Review of Factors Influencing Pain Sensation at the Injection Site. Advances in Therapy, 36(11), 2986–2996. doi: 10.1007/s12325-019-01101-6. Retrieved from https://pmc.ncbi.nlm.nih.gov/articles/PMC6822791/