Key takeaways
Mounjaro (tirzepatide) and Ozempic (semaglutide) are similar GLP-1 medications that are FDA-approved to treat type 2 diabetes.
Both medications can also produce significant weight loss and may be prescribed off-label for weight management.
While they share similarities, Mounjaro and Ozempic have some key differences, including slightly different mechanisms of action, active ingredients, dosing, and effectiveness.
Clinical trials and other studies suggest that Mounjaro outperforms Ozempic for weight loss and blood sugar control.
Here's what we'll cover
Key takeaways
Mounjaro (tirzepatide) and Ozempic (semaglutide) are similar GLP-1 medications that are FDA-approved to treat type 2 diabetes.
Both medications can also produce significant weight loss and may be prescribed off-label for weight management.
While they share similarities, Mounjaro and Ozempic have some key differences, including slightly different mechanisms of action, active ingredients, dosing, and effectiveness.
Clinical trials and other studies suggest that Mounjaro outperforms Ozempic for weight loss and blood sugar control.
Mounjaro and Ozempic are part of a blockbuster new class of injectable drugs that is significantly changing diabetes treatment. While clinical trials and real-world data have proven them to be highly effective for blood sugar control in people with type 2 diabetes, they may also have other health benefits, including significant weight loss for people with obesity and overweight.
If you’re considering trying one of these diabetes medications for weight loss or better blood sugar control, you may be curious about the differences between Mounjaro and Ozempic, including how they work, their results, safety, side effects, and more.
Read on to learn more about these two drugs and get a detailed comparison of Mounjaro versus Ozempic.
What are Mounjaro and Ozempic?
Mounjaro (tirzepatide)and Ozempic (semaglutide) belong to a class of drugs called glucagon-like peptide-1 receptor agonists, or GLP-1s. Both drugs are approved by the U.S. Food and Drug Administration (FDA) to help improve blood sugar control in people with type 2 diabetes. They may also be prescribed off-label for weight loss for people with obesity or overweight.
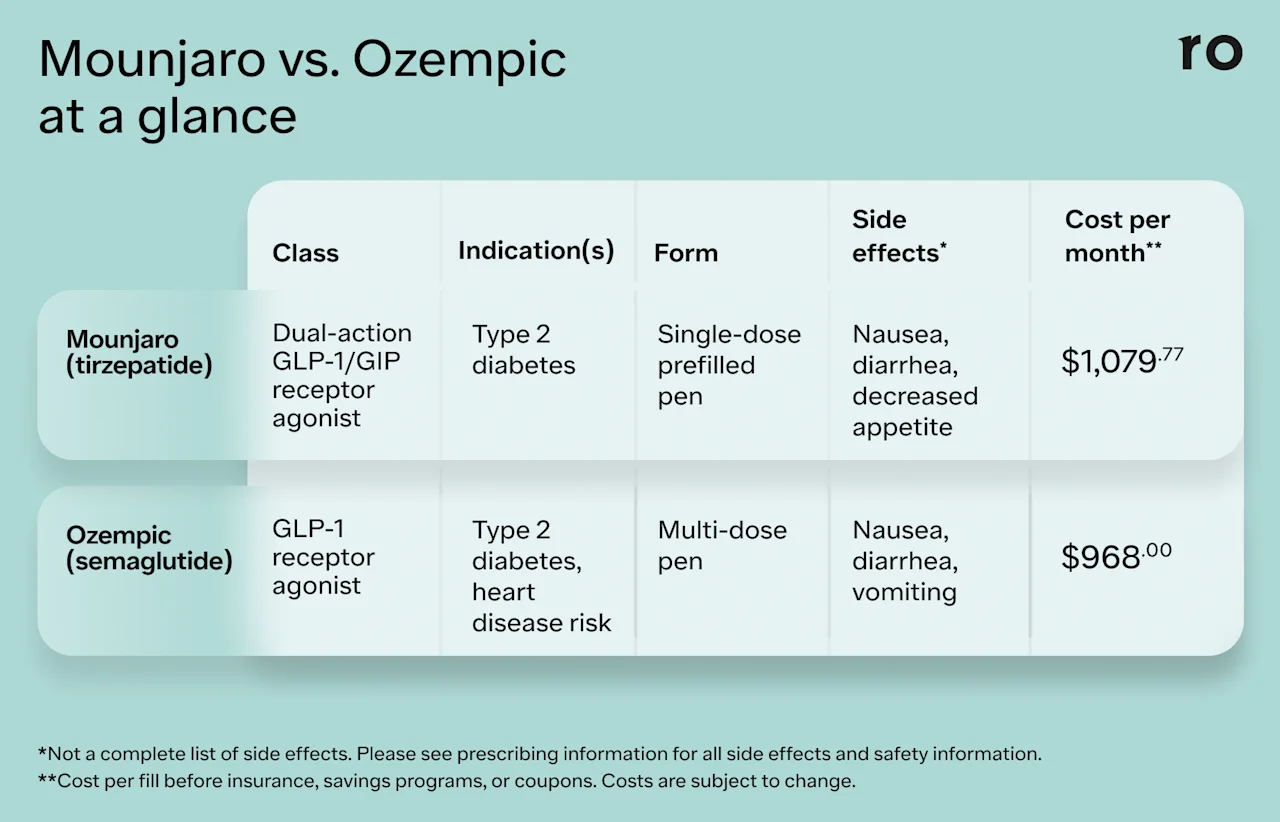
Here’s a quick look at some key details about each medication.
Mounjaro
Drug class: Mounjaro is a glucagon-like peptide-1 and a glucose-dependent insulinotropic polypeptide (GLP-1/GIP) receptor agonist. It contains the active ingredient tirzepatide.
Indication: Mounjaro is FDA-approved for the treatment of type 2 diabetes.
Form and frequency: Mounjaro is an injectable medication that you take once weekly. It comes in a prefilled, single-dose pen.
Dosing: Mounjaro comes in 2.5 mg, 5 mg, 7.5 mg, 10 mg, 12.5 mg, and 15 mg doses. The starting dose is 2.5 mg once a week for four weeks. Depending on how well you tolerate the medication, your healthcare provider may gradually increase your dose every four weeks until you reach a maintenance dose level, which varies from person to person.
Mechanism of action: Mounjaro works by mimicking the naturally occurring gut hormones GLP-1 and GIP (sometimes also called gastric inhibitory polypeptide). Because Mounjaro mimics two hormones, it has a dual mechanism of action and is also referred to as a dual agonist.
Ozempic
Drug class: Ozempic is a glucagon-like peptide-1 (GLP-1) receptor agonist. It contains the active ingredient semaglutide.
Indications: The FDA has approved Ozempic for the treatment of type 2 diabetes. It’s also approved to reduce the risk of major cardiovascular health problems, such as heart attack, stroke, and death in adults with type 2 diabetes and heart disease.
Form and frequency: Ozempic comes in a multi-dose, prefilled pen that you inject once per week.
Dosing: Ozempic comes in dose strengths of 0.25 mg, 0.5 mg, 1 mg, and 2 mg. Your healthcare provider will likely start you at the lowest dose (0.25 mg) for four weeks before increasing to the next dose strength based on how well you tolerate the medication. Your healthcare provider will typically increase your dose every four weeks until you reach your maintenance dose.
Mechanism of action: Ozempic works by mimicking the naturally occurring gut hormone GLP-1.
Tirzepatide and semaglutide, the active ingredients in Mounjaro and Ozempic, are also available under different brand names and for different indications:
Zepbound (tirzepatide) is approved for weight management in people with obesity or overweight with a weight-related health condition (such as high blood pressure). It is also approved for obstructive sleep apnea in adults with obesity.
Wegovy (semaglutide) is approved for weight management in people with obesity, and adults with overweight who have a weight-related comorbidity such as high cholesterol or type 2 diabetes. It’s also indicated to reduce the risk of life-threatening cardiovascular events (heart attack, stroke) in people with obesity and overweight who also have heart disease.
Rx weight loss with Ro
Get access to prescription weight loss medication online
How do Mounjaro and Ozempic work?
Both Mounjaro and Ozempic mimic naturally occurring incretin hormones, which are produced in the gastrointestinal tract. A key difference between these medications is that Mounjaro mimics GLP-1 and GIP, whereas Ozempic only mimics GLP-1.
In clinical trials, Mounjaro shows superior blood sugar control and weight loss results. More research is needed to determine whether the added effect of mimicking GIP in addition to GLP-1 is the reason why.
When we eat, the intestines release GLP-1, which has several functions in the body. GIP, also produced in the intestines, works in tandem with GLP-1 to help regulate appetite and stimulate insulin secretion after eating, which helps manage blood sugar levels and counteract insulin resistance.
Mounjaro and Ozempic work by binding to and activating these GLP-1 and GIP receptors in the body, which can help regulate blood sugar and promote weight loss by:
Improving insulin sensitivity. People with type 2 diabetes or obesity may also have insulin resistance, which occurs when cells stop responding as well to the hormone insulin. Insulin helps cells uptake glucose (sugar) to be used for energy. Insulin resistance can cause blood sugar levels to remain high and excess sugar to be stored in the body as fat. The GLP-1 hormone that Mounjaro and Ozempic mimic can help your pancreas produce more insulin. Increased insulin production can help lower blood sugar levels and counteract insulin resistance, which may help with weight loss. GLP-1 receptor agonists also reduce glucagon secretion. Glucagon is a hormone that raises blood sugar by signaling the liver to release stored glucose. This dual action of stimulating insulin and suppressing glucagon helps lower blood sugar more effectively.
Decreasing appetite. Our brain and gut communicate through what’s called the gut-brain axis. One function of this axis is to signal when we are full or almost full while eating. GLP-1 medications activate the GLP-1 receptors in the brain. This helps reduce appetite and quiet “food noise,” which is a nonmedical term that describes a type of intrusive preoccupation with food.
Slowing gastric emptying. GLP-1s help slow gastric emptying, which is the rate food travels from the stomach to the lower intestine. This may help you feel full earlier while eating meals and snacks, and help you feel satisfied for longer.
GLP-1 medications work best when combined with lifestyle changes to diet and physical activity levels. This includes eating a balanced diet and exercising for about 150 minutes weekly. Other lifestyle interventions, such as adequate hydration, good sleep, and stress management are also important for overall health.
While both drugs may have immediate health benefits, they are not short-term medications. Mounjaro and Ozempic are designed to be taken long-term—and sometimes indefinitely—to maintain blood sugar and weight loss results. Stopping GLP-1 treatment too soon can also lead to rebound weight gain or increased blood sugar levels.
Mounjaro vs. Ozempic: weight loss and A1C control
While both medications can be effective tools for type 2 diabetes and obesity, a mix of clinical trials and other research has found Mounjaro to be somewhat superior to Ozempic for weight loss and blood sugar control. Here are some examples.
Blood sugar control
In a 2021 clinical trial comparing the active ingredients of Mounjaro (tirzepatide) and Ozempic (semaglutide), researchers found tirzepatide to be more effective at lowering both blood sugar and weight in people with type 2 diabetes.
The trial, published in the New England Journal of Medicine, involved 1,878 participants with type 2 diabetes. The researchers randomly assigned participants in equal numbers to four groups: a once-weekly semaglutide injection of 1 mg or a once-weekly tirzepatide injection of either 5 mg, 10 mg, or 15 mg.
At the start of the trial, the participants had an average hemoglobin A1C (HbA1c) of 8.28%. HbA1c indicates a person’s average blood sugar level over about 90 days and is a common blood test to diagnose prediabetes or type 2 diabetes and monitor blood sugar control for people with these conditions. By the end of the 40-week trial, the estimated changes for A1C were -1.86% for those receiving 1 mg of semaglutide and -2.01%, -2.24%, and -2.30% for those receiving the respective tirzepatide doses of 5 mg, 10 mg, and 15 mg.
Ultimately, even one of the lowest doses of tirzepatide (5 mg) was more effective at lowering A1C than the second-to-highest dose of semaglutide (1 mg).
Weight loss
Weight loss results with Mounjaro and Ozempic can vary significantly, depending on dosage, adherence to lifestyle interventions, and other individual factors.
Mounjaro and Ozempic are not indicated for weight loss; however, since healthcare providers sometimes prescribe Mounjaro and Ozempic off-label for weight loss in people without type 2 diabetes, studies comparing tirzepatide and semaglutide in people with overweight and obesity are also important.
In the 2021 trial referenced above, participants receiving tirzepatide also had greater reductions in body weight than those on semaglutide. Those receiving 1 mg of semaglutide saw an average reduction of 5.7 kg (about 12.5 pounds) of body weight. Those receiving 5 mg, 10mg, and 15 mg experienced an average reduction of 7.6 kg (about 17 pounds), 9.3 kg (about 20 pounds), and 11.2 kg (about 25 pounds) respectively.
More recently, a 2024 cohort study looked at electronic health record data for 18,386 people who were taking one of the medications between May 2022 and September 2023. Of note, 52% of participants also had a type 2 diabetes diagnosis. Regardless of participants’ type 2 diabetes status, researchers found that people receiving tirzepatide injections lost about 7% more weight than those receiving semaglutide injections in the first year on the medication. However, both medications produced at least a 5% decrease in body weight for most of the cohort.
Mounjaro vs. Ozempic: side effects and risks
Mounjaro and Ozempic have similar safety profiles and are considered safe when used as directed under the supervision of an experienced healthcare provider.
Both drugs can cause mild-to-moderate gastrointestinal issues, though these tend to be manageable and go away as your body adjusts to the medication.
Some common side effects of Mounjaro include:
Nausea
Diarrhea
Decreased appetite
Vomiting
Constipation
Dyspepsia (indigestion)
Abdominal pain
The common side effects of Ozempic are similar and may include:
Abdominal pain
Constipation
Diarrhea
Nausea
Vomiting
Rare but serious side effects may also occur with both medications. These include:
Allergic reactions. Symptoms may include facial or throat swelling, difficulty breathing or swallowing, itching or rash, fainting or feeling dizzy, or rapid heartbeat.
Inflammation of the pancreas (pancreatitis). Symptoms may include severe abdominal pain that doesn’t go away and sometimes back pain.
Gallbladder problems. Symptoms may include pain in the upper abdomen, fever, yellowing of the skin or eyes, or clay-colored stools.
Kidney problems (kidney failure). People with existing kidney problems may notice worsening symptoms if they become chronically dehydrated.
Low blood sugar (hypoglycemia). Symptoms may include dizziness or lightheadedness, blurred vision, mood changes, sweating, slurred speech, hunger, confusion, drowsiness, shakiness, weakness, headache, rapid heartbeat, or feeling jittery.
Vision changes. Symptoms may include blurred vision, floaters, or spots. For people with type 2 diabetes who also have a history of diabetic retinopathy (a condition that can cause blood vessel damage in the retina), these medications may worsen symptoms. People with a known history of diabetic retinopathy should be closely monitored while taking these medications.
Gastroparesis or bowel obstruction. Some research has found that these meds may be associated with gastroparesis (stomach paralysis) and bowel obstruction. Symptoms can include bloating, abdominal pain, and vomiting.
Both drugs also carry a black box warning that they may increase the risk of thyroid C-cell tumors. While this type of cancer was only found in rodent studies (not in humans), people who have a personal or family history of thyroid cancer, including medullary thyroid carcinoma (MTC), or MEN2 syndrome shouldn't take GLP-1 medications like Mounjaro or Ozempic.
Mounjaro vs. Ozempic: cost and accessibility
The cost of these GLP-1 medications is also similar—both Mounjaro and Ozempic can cost more than $1,000 per month. However, what you end up paying for each drug may vary by pharmacy and insurance coverage. Most insurance plans, including Medicare and Medicaid, cover Mounjaro and Ozempic for their FDA-approved indication: type 2 diabetes management.
Getting coverage for off-label weight loss alone may be challenging, as most insurance providers don’t cover off-label drug use.
You may also be eligible for manufacturer savings programs and coupons, which may help reduce the monthly cost of these medications. However, some manufacturer savings programs and discounts may not apply to off-label use.
Mounjaro cost
The cost of Mounjaro is about $1,079.77 per month before insurance.
Eli Lilly (the manufacturer of Mounjaro) offers a Mounjaro Savings Card for people with commercial insurance coverage. With the savings card, you may pay as low as $25 per month if you meet eligibility requirements.
You may also be eligible for pharmacy-specific Mounjaro coupons through discount sites like GoodRx or SingleCare.
Ozempic cost
The current cost of Ozempic without insurance is $968.52.
The Ozempic Savings Card may help people with commercial or private insurance pay as little as $25 per month. Novo Nordisk (the maker of Ozempic) also has a Patient Assistance Program that can help people with type 2 diabetes who are uninsured and experiencing financial hardship access Ozempic for free.
Some discount sites may also offer Ozempic coupons at large retail pharmacies like Walgreens and CVS.
Mounjaro vs. Ozempic: similarities and differences
To compare Mounjaro and Ozempic, understanding their shared benefits and key differences is helpful. Here’s a quick recap of some of the information we’ve covered so far.
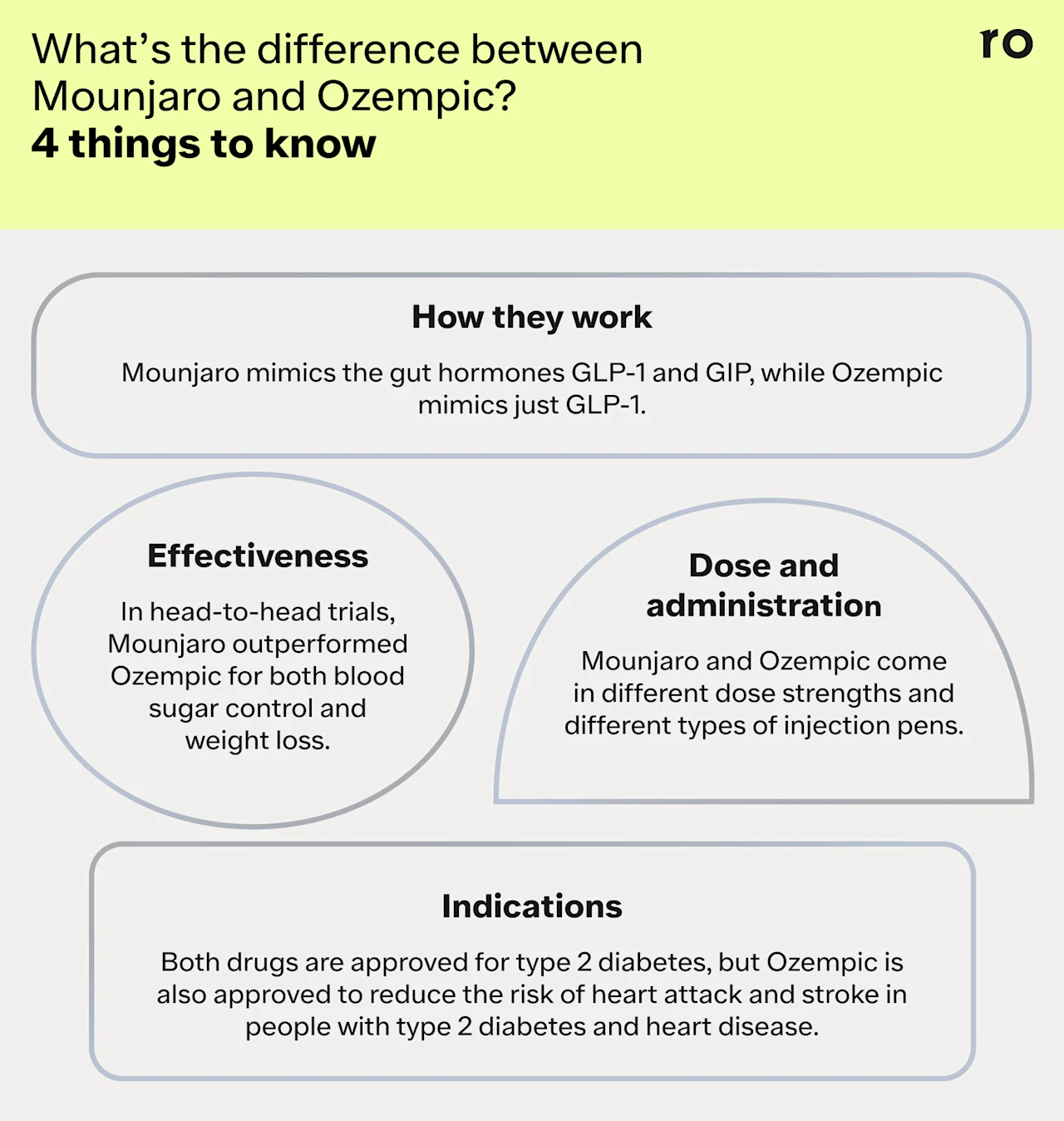
Similarities between Mounjaro and Ozempic
They’re both FDA-approved for type 2 diabetes and may be prescribed off-label for weight loss.
They’re both once-weekly subcutaneous injectables, meaning you inject them underneath the skin on the same day and time each week.
They both mimic the GLP-1 hormone.
They’re both long-term medications—people typically take Mounjaro and Ozempic for at least 12 weeks and up to a year or longer.
Both medications can be effective tools for blood sugar control and weight management.
They can both be expensive and may cost over $1,000 a month. Insurance, savings programs, or discounts can help mitigate out-of-pocket costs.
They share common GI side effects, such as nausea, diarrhea, and vomiting. Common side effects tend to be mild and may go away over time.
Differences between Mounjaro and Ozempic
They have different active ingredients: tirzepatide (Mounjaro) and semaglutide (Ozempic).
They have slightly different mechanisms of action. Mounjaro is a dual-agonist drug that targets both GLP-1 and GIP receptors. Ozempic only targets GLP-1.
They have different indications—while both drugs are approved to treat type 2 diabetes, Ozempic is also indicated to reduce the risk of heart attack and stroke in people with type 2 diabetes and heart disease.
They come in different dose strengths.
While both medications are injectable, they use different injection pens. Mounjaro is a single-dose pen that you dispose of after each weekly injection. Ozempic is a multi-dose pen, meaning you manually select your dose and attach a new needle each week.
Research suggests that Mounjaro may be more effective than Ozempic for blood sugar control and weight loss.
Mounjaro or Ozempic: which one is right for you?
Finding the right treatment depends on your underlying conditions, risk factors, weight, personal and family health history, and other factors. If you’re curious about starting a GLP-1 medication, the first step is to talk to your healthcare provider. You can also connect with a licensed healthcare professional through Ro.
Some questions you may want to ask include:
Am I a good candidate for GLP-1 treatment?
What are the possible side effects? How can I manage them?
Will my insurance cover my medication?
Which medication is more effective for blood sugar control?
Which medication is better for weight loss?
Do I have to change my diet while I’m on medication?
How will you monitor my progress or any side effects?
Your healthcare provider can evaluate your health history and help you consider each medication's potential pros and cons to find a treatment tailored to your needs and health goals.
Bottom line
While Mounjaro and Ozempic share some similarities, they are not the same and are not interchangeable medications.
Both Mounjaro and Ozempic are effective treatments for type 2 diabetes that can also promote significant weight loss in some people.
Ozempic and Mounjaro have slightly different mechanisms of action. Ozempic works by mimicking one naturally occurring gut hormone, while Mounjaro mimics two; otherwise these two medications are quite similar in how they work.
Both drugs may cause mild but manageable GI side effects, such as nausea, vomiting, and diarrhea.
In clinical trials and other studies, Mounjaro outperforms Ozempic for weight loss and blood sugar control, potentially because of its dual mechanisms of action.
Alternatives to Mounjaro and Ozempic for weight loss include Zepbound and Wegovy. These GLP-1 drugs are specifically indicated for weight loss.
Frequently asked questions (FAQs)
What is better for weight loss, Ozempic or Mounjaro?
Both Ozempic and Mounjaro have been shown to promote weight loss. However, in clinical trials and other studies, tirzepatide (the active ingredient in Mounjaro) outperforms semaglutide (the active ingredient in Ozempic) for weight loss.
Why do people switch from Ozempic to Mounjaro?
Some people may switch from Ozempic to Mounjaro (under the guidance of their healthcare provider) because of side effects or because they have heard that tirzepatide (the active ingredient in Mounjaro) is more effective than semaglutide (the active ingredient in Ozempic) for weight loss and lowering blood sugar.
Can I substitute Ozempic for Mounjaro?
Ozempic and Mounjaro, although similar, are different medications taken at different doses. You should not substitute one for the other. Talk to your prescribing healthcare provider if you’re interested in switching medications. They can help determine the best treatment based on your health history and overall goals.
What are the cons of taking Mounjaro?
The cons of taking Mounjaro may include potential gastrointestinal or other side effects, high cost if not covered by insurance, and the fact that it must be taken long-term for sustained results.
How much weight can you lose on Mounjaro in a month?
Weight loss on Mounjaro takes time. The amount and rate of weight loss on Moujaro will vary from person to person and may be impacted by factors like health history, starting weight, lifestyle interventions, and more. Some people may lose some weight within a month of starting the medication, but more time will be needed to continue the results.
In a recent cohort study, researchers found that patients lost, on average, about 3.6% of their body weight after taking tirzepatide (the active ingredient in Mounjaro) for three months. At six months, patients lost 10.1%, and at one year, they lost 15.3% of their body weight. Weight loss results will also depend on various factors, such as whether you’re taking the medication along with making lifestyle changes, your dosage, and other unique circumstances.
DISCLAIMER
If you have any medical questions or concerns, please talk to your healthcare provider. The articles on Health Guide are underpinned by peer-reviewed research and information drawn from medical societies and governmental agencies. However, they are not a substitute for professional medical advice, diagnosis, or treatment.
Mounjaro Important Safety Information: Read more about serious warnings and safety info.
Ozempic Important Safety Information: Read more about serious warnings and safety info.
GLP-1 Important Safety Information: Read more about serious warnings and safety info.
Zepbound Important Safety Information: Read more about serious warnings and safety info.
Wegovy Important Safety Information: Read more about serious warnings and safety info.
Baggio, L. L. & Drucker, D. J. (2014). Glucagon-like peptide-1 receptors in the brain: controlling food intake and body weight. The Journal of Clinical Investigation, 124(10), 4223. doi: 10.1172/JCI78371. Retrieved from https://pmc.ncbi.nlm.nih.gov/articles/PMC4191040/
Collins, L. & Costello, R. A. (2024). Glucagon-Like Peptide-1 Receptor Agonists. StatPearls. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK551568/
Freeman, A. M., Acevedo, L. A., & Pennings, N. (2025). Insulin resistance. StatPearls. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK507839/
Frías, J. P., Davies, M. J., Rosenstock, J., et al. (2021). Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. New England Journal of Medicine, 385(6), 503-515. doi: 10.1056/NEJMoa2107519. Retrieved from https://www.nejm.org/doi/full/10.1056/NEJMoa2107519
Gołacki, J., Matuszek, M., & Matyjaszek-Matuszek, B. (2022). Link between insulin resistance and obesity-from diagnosis to treatment. Diagnostics (Basel), 12(7), 1681. doi: 10.3390/diagnostics12071681. Retrieved from https://pubmed.ncbi.nlm.nih.gov/35885586/
Jensterle, M., Ferjan, S., Ležaič, L., et al. (2023). Semaglutide delays 4-hour gastric emptying in women with polycystic ovary syndrome and obesity. Diabetes, Obesity, and Metabolism, 25(4), 975-984. doi: 10.1111/dom.14944. Retrieved from https://pubmed.ncbi.nlm.nih.gov/36511825/
Harris, E. (2024). EHR data suggest tirzepatide outdoes semaglutide for weight loss. JAMA, 332(8), 610. doi: 10.1001/jama.2024.13770. Retrieved from https://jamanetwork.com/journals/jama/article-abstract/2822005
Hayashi, D., Edwards, C., Emond, J. A., et al. (2023). What is food noise? A conceptual model of food cue reactivity. Nutrients, 15(22), 4809. doi: 10.3390/nu15224809. Retrieved from https://pmc.ncbi.nlm.nih.gov/articles/PMC10674813/
Rodriguez, P. J., Goodwin Cartwright, B. M., Gratzl, S., et al. (2024) Semaglutide vs tirzepatide for weight loss in adults with overweight or obesity. JAMA Internal Medicine, 184(9), 1056. doi: 10.1001/jamainternmed.2024.2525. Retrieved from https://pubmed.ncbi.nlm.nih.gov/38976257/
Samms, R. J., Coghlan, M. P., & Sloop, K. W. (2020). How may gip enhance the therapeutic efficacy of glp-1? Trends in Endocrinology & Metabolism, 31(6), 410-421. doi: 10.1016/j.tem.2020.02.006. Retrieved from https://pubmed.ncbi.nlm.nih.gov/32396843/
Sodhi, M., Rezaeianzadeh, R., Kezouh, A., et al. (2023). Risk of gastrointestinal adverse events associated with glucagon-like peptide-1 receptor agonists for weight loss. JAMA, 330(18), 1795. doi: 10.1001/jama.2023.19574. Retrieved from https://pmc.ncbi.nlm.nih.gov/articles/PMC10557026/