Key takeaways
While Ozempic (semaglutide)is considered safe, some people may experience mild side effects. These side effects are often temporary and may improve as your body adjusts to the medication.
The most common side effects of Ozempic are gastrointestinal (GI) complaints like nausea, diarrhea, vomiting, abdominal discomfort, constipation, and acid reflux.
Ozempic may also cause more serious side effects, such as pancreatitis and allergic reactions, though these are rare.
Starting at the lowest dosage, eating smaller meals, and avoiding certain foods may help minimize common side effects.
Here's what we'll cover
Key takeaways
While Ozempic (semaglutide)is considered safe, some people may experience mild side effects. These side effects are often temporary and may improve as your body adjusts to the medication.
The most common side effects of Ozempic are gastrointestinal (GI) complaints like nausea, diarrhea, vomiting, abdominal discomfort, constipation, and acid reflux.
Ozempic may also cause more serious side effects, such as pancreatitis and allergic reactions, though these are rare.
Starting at the lowest dosage, eating smaller meals, and avoiding certain foods may help minimize common side effects.
If you're considering starting Ozempic (semaglutide), you may be wondering if it’s safe, if there are any side effects to know about, and what to expect in the first few weeks of treatment.
Ozempic comes in four dose strengths: 0.25 mg, 0.5 mg, 1 mg, and 2 mg. Most healthcare providers start patients on the lowest dose and gradually increase it every four weeks.
Ozempic is generally well-tolerated, but it can cause some mild side effects, which might be more noticeable when you first start taking it or when you move to a higher dose strength. The most common ones include gastrointestinal (GI) issues like nausea, vomiting, constipation, heartburn, and diarrhea. The good news is that these common GI symptoms tend to be mild and manageable and typically resolve as your body adjusts to the medication.
While not everyone who takes Ozempic will experience these symptoms, understanding these potential side effects is essential for using Ozempic safely and maximizing its benefits—whether you’re taking it for type 2 diabetes or have been prescribed Ozempic off-label for weight loss.
Read on to learn more about the most common side effects of Ozempic, tips to minimize symptoms, and when to seek medical advice.
Nausea
Nausea is one of the most commonly reported side effects of Ozempic. According to clinical trial data, 15.8% of patients on 0.5 mg and 20.3% of those on 1 mg of Ozempic experienced nausea compared to 6.1% of the placebo group.
Ozempic stimulates GLP-1 receptors in the pancreas, digestive tract, and central nervous system. By activating receptors in the stomach, Ozempic can slow down gastric emptying (the rate at which food moves from your stomach to your lower intestine). This means that food may stay in the stomach longer or take more time to digest.
"This can cause nausea and vomiting, especially if someone overeats or eats too quickly,” says Marlena Klein, DO, a board-certified physician in Internal Medicine.
Tips for managing nausea
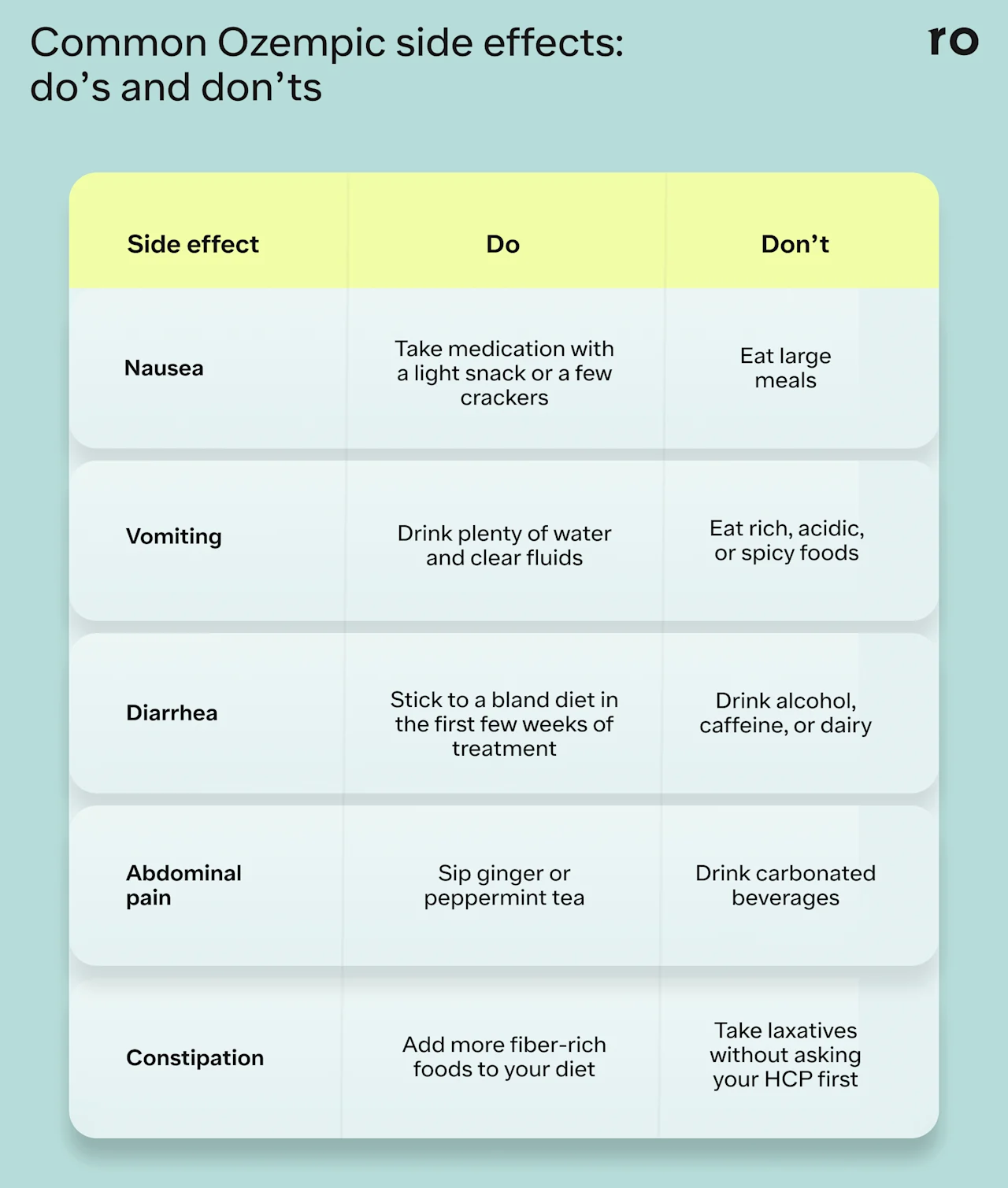
Limit rich, fatty, or spicy foods that may cause discomfort.
Eat smaller, more frequent meals to prevent overeating.
Take the medication with a light snack, like a few crackers.
Sip on water, ginger tea, or clear broths to soothe your stomach.
Dr. Klein also advises patients to inject their medication on a day with few commitments the following day. "Some patients find that injecting in the evening helps reduce nausea," she says, noting that light carbohydrates like crackers can help settle the stomach.
Vomiting
Vomiting is more common at higher doses of Ozempic. In clinical trials, about 5% of participants on 0.5 mg and 9.2% on 1 mg experienced vomiting, compared to 2.3% in the placebo group. In another study looking at treatment with 2.4 mg of semaglutide, roughly 24% of participants experienced vomiting compared to about 6% in the placebo group.
“I always tell patients at baseline to expect some nausea or vomiting the day after injecting, but it should never be debilitating,” says Dr. Klein.
Tips for managing vomiting
Sit upright or stand after eating to help prevent reflux.
Avoid high-fat, acidic, or spicy foods.
Limit alcohol or other carbonated beverages that can irritate the stomach.
Stay hydrated with electrolyte-rich fluids and water.
Dr. Klein also recommends anti-nausea or antiemetic medications for relief.
"Ondansetron (Zofran) can be effective during the first 1–2 days," she says. "However, if nausea or vomiting is severe, persistent, or doesn’t improve, patients should contact their provider."
Diarrhea
Diarrhea is another common side effect, and like others on this list, it tends to be worse when a person first starts taking Ozempic or when they switch to a higher dose. In one clinical trial, diarrhea occurred in 13% of patients taking 0.5 mg of Ozempic, 11% in those taking 1 mg, and 2% in the placebo group. In another study, about 31% of participants taking 2.4 mg of semaglutide experienced diarrhea.
It’s not entirely clear why Ozempic may cause diarrhea, but some experts believe it may be connected to semaglutide’s effects on digestion. One theory is that delayed gastric emptying may also affect the body’s absorption of certain nutrients, potentially leading to diarrhea.
Tips for managing diarrhea
Stay hydrated with clear fluids like water, tea, or broth to prevent dehydration.
Stick to a bland diet, like the BRAT diet, until symptoms subside.
Avoid caffeine, alcohol, spicy foods, and dairy, as these can exacerbate symptoms.
Use over-the-counter (OTC) medications to help control symptoms, but consult your healthcare provider first.
Dr. Klein also recommends fiber powder supplements such as Benefiber or Metamucil.
“If a patient has diarrhea the day or two after injection, I recommend starting the fiber powder one or two days before their injection to help bulk up their stool and reduce their diarrhea,” she says.
Constipation
Ozempic may cause constipation, although it is less common than side effects like nausea or diarrhea, with roughly 4–12% of people experiencing symptoms. That said, constipation may last longer than other common side effects—in one study, the median duration of constipation was 47 days or about six weeks.
Tips for managing constipation
Drink plenty of water throughout the day to help support digestion.
Engage in regular physical activity— exercise can help stimulate bowel movements.
Increase fiber intake with foods like fruits, vegetables, and whole grains.
Talk to your healthcare provider about using a stool softener or mild laxative.
Abdominal pain
Ozempic can also cause abdominal pain (bloating, cramping, or general discomfort), which may also be due to the medication’s impact on digestion. About 5-7% of clinical trial participants experienced abdominal pain.
Addressing underlying issues like nausea or vomiting may help ease abdominal pain. However, if abdominal pain becomes severe and doesn’t go away, or if the pain starts to radiate toward your back, stop taking Ozempic and seek medical care right away. These symptoms could be a sign of pancreatitis, a rare but serious adverse reaction to Ozempic.
Tips for managing mild abdominal pain or discomfort
Eat smaller meals and drink plenty of fluids to help aid with digestion.
Eat bland foods, like crackers or toast, and avoid heavy or spicy foods until symptoms subside.
Try drinking ginger or peppermint tea, which may help relieve discomfort or bloating.
Acid reflux and gas
Other GI side effects may include gas (burping, flatulence), indigestion, and acid reflux, though these are less common than side effects like nausea and vomiting.
In clinical trials, about 2% of participants reported gastroesophageal reflux disease (GERD)at lower doses of Ozempic. Dyspepsia (indigestion) also appeared to be more common at lower doses, with about 3.5% of participants reporting this symptom. Flatulence was more common with higher doses of Ozempic, but fewer than 2% of clinical trial participants reported this side effect.
Tips for managing acid reflux and gas
Avoid lying down after eating—try to eat 2-3 hours before bedtime.
Eat smaller meals throughout the day.
Prop up your head or sleep upright to prevent acid reflux at night.
Ask your healthcare provider about prescription or OTC remedies for heartburn.
Dr. Klein also suggests avoiding common acid reflux triggers like:
Onions
Alcohol
Chocolate
Citrus fruits
Spicy foods
Peppermint
Tomato sauce
Fatigue
Fatigue is a less common side effect of Ozempic, but some people may experience tiredness when they first start the medication or when they move to higher dose strengths.
It’s not clear why Ozempic may cause fatigue, but it may be due to the medication’s impact on appetite and blood sugar—the combined effect of lower calorie intake and lower blood sugar may cause some fatigue as your body adjusts to the medication.
Tips for managing fatigue
Consider taking your medication at night to help combat daytime sleepiness.
Eat small meals every few hours to help keep your blood sugar levels stable.
Prioritize rest and stay hydrated to help combat fatigue.
Try to maintain a consistent sleep schedule for better quality rest.
Dizziness
Dizziness is another less common side effect that may also be a symptom of low blood sugar. Ozempic can help lower blood sugar (even if you’re taking it for weight loss), so it’s important to let your healthcare provider know if you experience any signs or symptoms of low blood sugar—such as dizziness, weakness, confusion, or rapid heart rate—while you’re taking Ozempic.
Tips for managing dizziness
Monitor your blood sugar regularly to avoid extreme highs or lows.
If you feel dizzy, sit or lie down until it passes to prevent falls or injury.
Eat small, frequent meals throughout the day to help keep blood sugar levels steady.
Avoid sudden movements, such as standing up too quickly, which can worsen dizziness.
Injection site reactions
Ozempic is injected subcutaneously (under the skin) each week in the abdomen, thigh, or upper arm. Some people may experience minor skin irritation at the injection site, such as itching, swelling, or a rash. However, hypersensitivity reactions are rare.
Tips for managing injection site reactions
Have your provider show you how to inject Ozempic to avoid potential infection or irritation.
Avoid injecting into areas with irritation, bruising, or scarring.
Try rotating your injection sites each week to minimize irritation and discomfort.
Use a cold compress to help soothe the area if you notice swelling or redness.
Rare but serious side effects
Ozempic is generally safe, but it may cause some rare but serious side effects. If you experience any of the symptoms listed below, stop taking Ozempic and seek immediate medical attention.

Pancreatitis
Pancreatitis (inflammation of the pancreas) is very rare with Ozempic but has been reported in clinical trials. This side effect may be more common in people with certain health conditions or who have other risk factors for pancreatitis, including gallstones, alcohol use disorder, or a family history of pancreatitis.
Pancreatitis is a serious health issue. Seek immediate medical attention if you experience the following symptoms:
Severe pain in the upper abdomen that may radiate to your back
Nausea or vomiting
Fever
Fast heart rate
Thyroid Tumors
Ozempic has a black box warning for the potential risk of certain types of thyroid tumors, including medullary thyroid carcinoma. The risk of developing thyroid cancer from Ozempic is rare—a 2024 review of 10 studies involving 14,550 participants found that the incidence of thyroid cancer in people treated with semaglutide was under 1%.
That said, people with a personal or family risk of thyroid cancer should avoid Ozempic.
Symptoms of thyroid tumors may include:
A lump in the neck
Difficulty swallowing
Sudden hoarseness
Shortness of breath
Consult your healthcare provider as soon as possible for further evaluation if you experience any symptoms of potential thyroid issues.
Kidney Issues
In rare cases, Ozempic can affect kidney function. However, Dr. Klein says that if used correctly and tolerated well, Ozempic may improve kidney health. In January 2025 Ozempic was approved by the US Food and Drug Administration (FDA) to reduce the risk of worsening kidney disease and kidney failure in people with type 2 diabetes who also have chronic kidney disease.
“Uncontrolled blood sugars can worsen a patient's kidney function over time and is a leading cause of chronic kidney disease,” she says. “By controlling blood sugars, Ozempic can improve kidney function.”
It’s important to monitor blood sugar levels regularly and follow your healthcare provider's guidance on dosage while taking Ozempic.
Symptoms of possible kidney injury may include:
Changes in urination
Swelling in your feet, ankles, or legs
Fatigue or weakness
Gallbladder Problems
A 2022 review of research on GLP-1 agonists found that these medications may increase the risk of gallstones and gallbladder infections. The risk was higher with higher doses and longer treatment durations.
Symptoms of gallstones or gallbladder inflammation may include:
Sudden, intense abdominal pain
Pain in the right shoulder
Nausea
Vomiting
Fever
Allergic Reactions
While rare, Ozempic may cause allergic reactions in some individuals. Mild symptoms can include:
Rash
Itching
Flushing (temporary warmth, redness, or darkening of the skin)
If you experience mild allergic reactions, such as rash, itching, or flushing, inform your healthcare provider as soon as possible.
In some instances, Ozempic could cause more severe allergic reactions (including anaphylaxis). Seek immediate medical attention if you experience any symptoms of a serious allergic reaction, including:
Swelling of the eyes, face, mouth, tongue, or throat
Hives or itchy skin
Wheezing or difficulty breathing
Lightheadedness
Changes in pulse (rapid or weak pulse)
Hypoglycemia
Ozempic carries a very low risk of hypoglycemia, particularly in people who do not have diabetes. However, low blood sugar can occur while using Ozempic. The risk may be higher if you're taking other medications that also lower blood sugar levels, such as insulin or sulfonylurea drugs.
Symptoms of hypoglycemia may include:
Sweating
Confusion
Shakiness
Pallor (paleness)
Impaired cognition
Increased heart rate
Raised blood pressure
Vision Changes
In rare instances, some people may notice changes in their vision while taking Ozempic. For example, people with diabetic retinopathy—a complication of type 2 diabetes that damages the blood vessels in the retina—may experience worsening symptoms. It’s not entirely clear why this happens, but it may be due to changes in blood sugar control.
Symptoms of diabetic retinopathy may include:
Blurry vision
Dark spots
Floaters
Vision loss
Who should not take Ozempic?
It's essential to consult with your healthcare provider before starting Ozempic to ensure it's safe for you. You may not be eligible for Ozempic if you:
Are pregnant or breastfeeding
Have a history of diabetic retinopathy
Have or have had problems with your pancreas or kidneys
Have a personal or family history of medullary thyroid carcinoma (MTC)or multiple endocrine neoplasia syndrome type 2 (MEN 2)
Have a history of severe allergic reactions to semaglutide or other GLP-1 medications
Potential medication interactions
Ozempic can interact with some medications. Talk to your healthcare provider about any medications you’re taking (including prescriptions, OTC drugs, and supplements) so they can determine if any of your current medications might interact with Ozempic.
This may include:
Oral contraceptives. While Ozempic itself does not interfere with oral birth control, certain side effects might. For example, vomiting may reduce the effectiveness of birth control pills.
Blood pressure medications. Ozempic may help lower blood pressure in certain people. While this is generally a positive health benefit, people who take blood pressure meds may need to reduce the dosage while they’re on Ozempic, and will need regular monitoring to ensure their blood pressure doesn’t drop too low.
Other GLP-1 receptor agonists. Using Ozempic with other GLP-1 drugs is not advised—there are no additional health benefits from combining these drugs and doing so can increase your risk of severe side effects.
Insulin or other diabetes medications. If you take insulin or other diabetes medications, your provider may need to adjust your doses to prevent dangerously low blood sugar.
Dr. Klein adds that people who are on medications that need to be taken on an empty stomach should talk with their healthcare provider about regular monitoring.
"The medication's absorption can be affected by slowed gastric motility,” she says. “If food is still in the stomach, it can reduce the medication's effectiveness.”
Managing Ozempic side effects: general tips
If you’re just getting started on Ozempic, try these tips to help minimize your risk for common side effects:
Start at the lowest dose. Follow your provider’s guidance to start at the lowest dose and then slowly increase it over time, depending on how well you tolerate Ozempic. Gradual dose escalation can help minimize GI symptoms like nausea or abdominal pain.
Stay hydrated. Drink plenty of water or other clear fluids throughout the day. This can help you stay hydrated and can also aid in digestion.
Eat smaller portions. Eat smaller, more frequent meals to help your digestive system adjust to the medication. In the first few weeks of treatment, you may want to stick to a bland diet to help with any nausea or diarrhea.
Monitor blood sugar. If you have diabetes, check your blood sugar levels regularly to help mitigate complications from low blood sugar.
Stay in touch with your healthcare provider. Contact your provider to discuss any side effects you experience and raise any questions or concerns you might have.
When to seek medical help for Ozempic side effects
You may experience common GI side effects when you first start taking Ozempic, but these usually go away as your body adjusts.
If the side effects don’t improve or get worse, don’t hesitate to contact your healthcare provider. "These medications are supposed to help, not cause more harm," Dr. Klein emphasizes.
Contact your provider right away if you experience:
Dizziness
Chronic fatigue
Severe vomiting
Severe diarrhea
Severe dehydration
Persistent abdominal pain or cramping
Persistent or severe nausea
Swelling in the legs or ankles
Consistent headaches or migraine
Difficulty breathing or shortness of breath
Are Ozempic side effects worth it?
Ozempic can effectively control blood sugar and promote weight loss, but some people may experience side effects that could affect their daily routine.
For many, the potential health benefits outweigh the potential side effects.
Talk to your healthcare provider if you’re concerned about how Ozempic might affect you. They can help you understand the potential side effects, track your progress, and determine if Ozempic is the right option for your health goals, medical history, and other conditions. They may also recommend alternative medications that might be better suited to your health needs and goals.
Hear from Ro patients
Ro members taking branded GLP-1 medications were paid for their testimonials.
Bottom line
Ozempic is an effective and safe medication, but it may cause GI issues when you first start taking it or when you move up to a higher dose. These side effects are generally mild and manageable and tend to improve as your body adjusts, typically within a few weeks.
The most common side effects include nausea, vomiting, diarrhea, constipation, and abdominal pain.
Eating a bland diet, avoiding certain foods, staying hydrated, and taking anti-nausea medicine or fiber supplements may help minimize common side effects.
Serious side effects are rare but may include pancreatitis, kidney issues, and thyroid tumors. Seek immediate medical attention if you experience unusual symptoms, including shortness of breath, swelling, or severe abdominal pain.
Talk to your provider about any side effects you might experience, even if they are mild. They can help you find the best approach for managing discomfort.
Frequently asked questions (FAQs)
What are the downsides of Ozempic?
Ozempic may cause side effects such as nausea, vomiting, diarrhea, and constipation. Additionally, there are potential risks for rare but more serious concerns, including pancreatitis and kidney issues.
Do you gain weight back after stopping Ozempic?
Possibly. Once you stop taking Ozempic, your appetite and food cravings may return, and you may gain back some or most of the weight you lost.
Does Ozempic face go away?
“Ozempic face” is a non-medical term for facial sagging or hollowing that may be caused by rapid weight loss, but is not a side effect of Ozempic. Topical skincare or other cosmetic treatments may help improve skin texture and appearance. However, the extent of recovery may vary depending on factors like age, skin elasticity, and the amount of weight loss.
How long do the side effects of Ozempic last?
Side effects typically last several weeks after starting the medication or moving to a higher dose strength, but may persist longer in some people.
Can Ozempic cause heart palpitations?
Ozempic may cause a slight increase in heart rate. In clinical trials, 0.5 mg or 1 mg doses of Ozempic caused an increase of 2-3 heartbeats per minute in some participants. While this may not be concerning for most people, it’s important to have regular check-ins with your healthcare provider so they can monitor your overall health and progress while you’re taking Ozempic.
DISCLAIMER
If you have any medical questions or concerns, please talk to your healthcare provider. The articles on Health Guide are underpinned by peer-reviewed research and information drawn from medical societies and governmental agencies. However, they are not a substitute for professional medical advice, diagnosis, or treatment.
Ozempic Important Safety Information: Read more about serious warnings and safety info.
GLP-1 Important Safety Information: Read more about serious warnings and safety info.
Al-Sadawi, M. A., Aslam, F. M., Tao, M., et al. (2023). Effects of GLP-1 agonists on mortality and arrhythmias in patients with Type II diabetes. International Journal of Cardiology. Heart & Vasculature, 47, 101218. https://doi.org/10.1016/j.ijcha.2023.101218. Retrieved from https://www.sciencedirect.com/science/article/pii/S2352906723000490?via%3Dihub
Feier, C. V. I., Vonica, R. C., Faur, A. M., et al. (2024). Assessment of thyroid carcinogenic risk and safety profile of GLP1-RA semaglutide (Ozempic) therapy for diabetes mellitus and obesity: A systematic literature review. International Journal of Molecular Sciences, 25(8), 4346. https://doi.org/10.3390/ijms25084346. Retrieved from https://www.mdpi.com/1422-0067/25/8/4346
Gorgojo-Martínez, J. J., Mezquita-Raya, P., Carretero-Gómez, J., et al. (2022). Clinical Recommendations to Manage Gastrointestinal Adverse Events in Patients Treated with Glp-1 Receptor Agonists: A Multidisciplinary Expert Consensus. Journal of Clinical Medicine, 12(1), 145. https://doi.org/10.3390/jcm12010145. Retrieved from https://www.mdpi.com/2077-0383/12/1/145
He, L., Wang, J., Ping, F., et al. (2022). Association of glucagon-like peptide-1 receptor agonist use with risk of gallbladder and biliary diseases: A systematic review and meta-analysis of randomized clinical trials. JAMA Internal Medicine, 182(5), 513–519. doi: 10.1001/jamainternmed.2022.0338. Retrieved from https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2790392
Jones, M. W., Weir, C. B., & Ghassemzadeh, S. (2024). Gallstones (Cholelithiasis). StatPearls. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK459370/
Kennedy, C., Hayes, P., Salama, S., et al. (2023). The effect of semaglutide on blood pressure in patients without diabetes: A systematic review and meta-analysis. Journal of Clinical Medicine, 12(3), 772. https://doi.org/10.3390/jcm12030772. Retrieved from https://www.mdpi.com/2077-0383/12/3/772
Kommu, S. & Whitfield, P. (2024). Semaglutide. StatPearls. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK603723/
Leehey, D. J., Rahman, M. A., Borys, E., et al. (2021). Acute kidney injury associated with semaglutide. Kidney Medicine, 3(2), 282–285. doi: 10.1016/ j.xkme.2020.10.008. Retrieved from https://www.kidneymedicinejournal.org/article/S2590-0595(20)30269-7/fulltext
MedlinePlus. (n.d.). Diabetes and eye disease. U.S. National Library of Medicine. Retrieved from https://medlineplus.gov/ency/article/001212.htm
Novo Nordisk. (n.d.). Important safety information | Ozempic® (semaglutide) injection. Retrieved from https://www.ozempic.com/important-safety-information.html
Novo Nordisk. (2021). Ozempic (semaglutide) injection, for subcutaneous use: Full prescribing information. Retrieved from https://rsc.niaid.nih.gov/sites/default/files/1.14.2.2-package-insert-ozempic.pdf
Novo Nordisk. (2025). Ozempic (semaglutide) injection, for subcutaneous use: Prescribing information. Retrieved from https://www.novo-pi.com/ozempic.pdf
Patel, F., Gan, A., Chang, K., & Vega, K. J. (2023). Acute pancreatitis in a patient taking semaglutide. Cureus, 15(8), e43773. doi: 10.7759/cureus.43773. Retrieved from https://www.cureus.com/articles/178616-acute-pancreatitis-in-a-patient-taking-semaglutide#!/
Rayas, M. S. & Salehi, M. (2024). Non-diabetic hypoglycemia. [Updated 2024 Jan 27]. In: Feingold, K. R., Anawalt, B., Blackman, M. R., et al. (Eds.), Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK355894/
Shu, Y., He, X., Wu, P., et al. (2022). Gastrointestinal adverse events associated with semaglutide: A pharmacovigilance study based on FDA adverse event reporting system. Frontiers in Public Health, 10, 996179. doi: 10.3389/fpubh.2022.996179. Retrieved from https://pmc.ncbi.nlm.nih.gov/articles/PMC9631444/
Smits, M. M. & Van Raalte, D. H. (2021). Safety of semaglutide. Frontiers in Endocrinology, 12, 645563. doi: 10.3389/fendo.2021.645563. Retrieved from https://pubmed.ncbi.nlm.nih.gov/34305810/
Sorli, C., Harashima, S. I., Tsoukas, G. M., et al. (2017). Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. The Lancet. Diabetes & Endocrinology, 5(4), 251–260. doi: 10.1016/S2213-8587(17)30013-X. Retrieved from https://pubmed.ncbi.nlm.nih.gov/28110911/
Wharton, S., Calanna, S., Davies, M., et al. (2022). Gastrointestinal tolerability of once-weekly semaglutide 2.4 mg in adults with overweight or obesity, and the relationship between gastrointestinal adverse events and weight loss. Diabetes, Obesity & Metabolism, 24(1), 94–105. doi: 10.1111/dom.14551. Retrieved from https://pmc.ncbi.nlm.nih.gov/articles/PMC9293236
Wilding, J. P. H., Batterham, R. L., Calanna, S., et al. (2021). Once-Weekly Semaglutide in Adults with Overweight or Obesity. The New England Journal of Medicine, 384(11), 989–1002. doi: 10.1056/NEJMoa2032183. Retrieved from https://www.nejm.org/doi/10.1056/NEJMoa2032183













