Here's what we'll cover
Here's what we'll cover
Type 2 diabetes is a complex disease that until recently, was pretty difficult to treat. Some medications help lower blood sugar, but may not help with symptoms like increased appetite or body weight gain.
Thankfully, newer drugs like semaglutide help with all of these concerns. For some time, semaglutide was only available as an injection—until Rybelsus entered the picture.
What is Rybelsus?
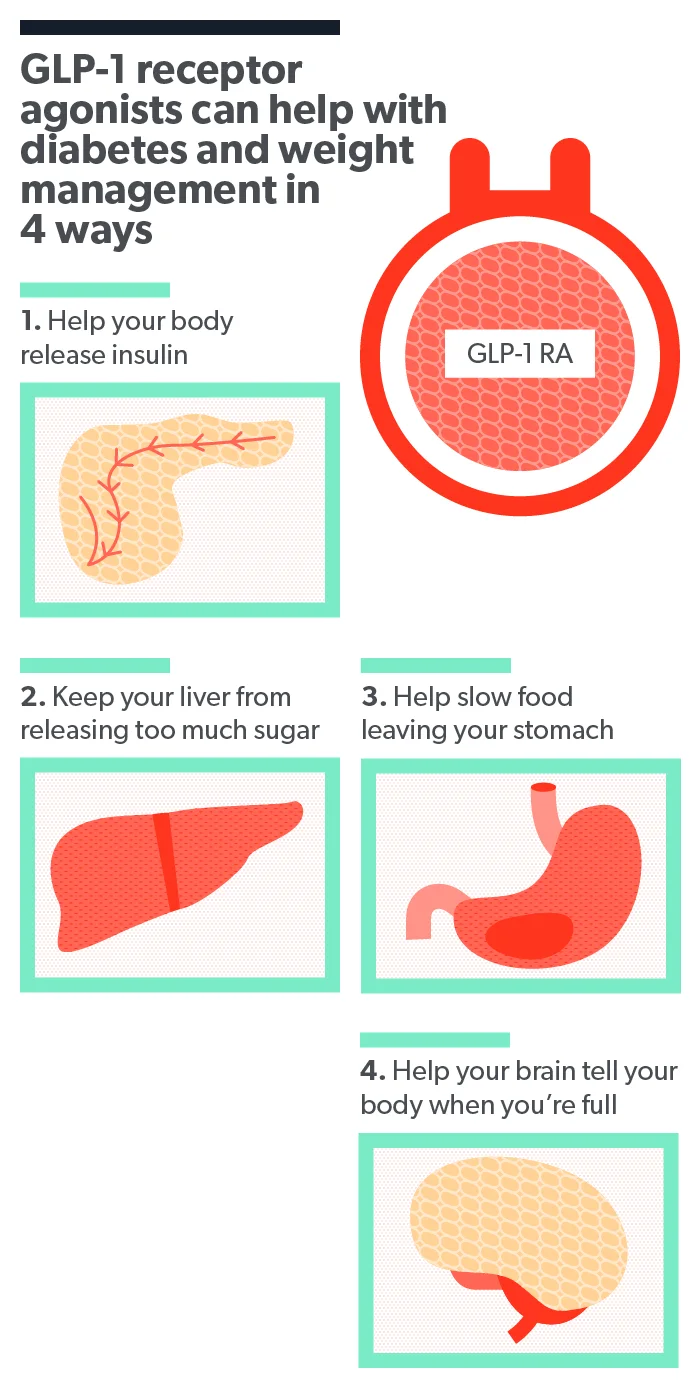
Rybelsus is the oral form of the drug semaglutide. It’s a type of medication called a glucagon-like peptide-1 receptor agonist, or GLP-1 for short. Other formulations of semaglutide include the injectable drugs Wegovy and Ozempic .
GLP-1s work by attaching to GLP-1 receptors in your pancreas, digestive tract, and brain. This encourages the pancreas to release more insulin, which is a hormone that helps lower blood sugar (glucose). At the same time, it emits less of a hormone that raises blood sugar called glucagon.
Medications like Rybelsus also bind to GLP-1 receptors involved in regulating appetite, like your brain and digestive tract. This makes it so you won’t feel hungry all the time (Shaefer, 2015).
GLP-1 Important Safety Information: Read more about serious warnings and safety info.
Wegovy Important Safety Information: Read more about serious warnings and safety info.
Ozempic Important Safety Information: Read more about serious warnings and safety info.
Rybelsus uses
Rybelsus is approved by the U.S. Food and Drug Administration (FDA) to treat type 2 diabetes mellitus (FDA, 2019). One of the primary features of type 2 diabetes is high blood sugar.
In clinical trials, people with type 2 diabetes who took Rybelsus showed significantly lower blood sugar levels on standard hemoglobin A1C tests than those who took a placebo (Anderson, 2020; Davies, 2017).
Some providers prescribe semaglutide off-label for weight management. Clinical trials have shown the drug helps with weight loss in people with type 2 diabetes. Many people with diabetes achieve healthier blood sugar levels after losing weight (Granhall, 2019; Pratley, 2019).
So far only one brand of semaglutide––an injectable drug called Wegovy––is FDA-approved for weight loss.
Side effects of Rybelsus
Rybelsus affects your stomach and intestines, so most of its side effects are related to the digestive system. Common side effects include stomach upset, nausea, vomiting, constipation, and decreased appetite (FDA, 2017).
Serious side effects of Rybelsus are less common and include (FDA, 2017):
Low blood sugar: Rybelsus helps lower blood sugar, but sometimes it overdoes it, resulting in hypoglycemia. This is more common if you’re taking other medications that control blood sugar at the same time as semaglutide.
Inflammation of your pancreas (pancreatitis): Some signs of this condition are severe abdominal pain that feels like it goes through to your back and uncontrollable vomiting. Pancreatitis can be very dangerous if it's not treated quickly. That said, this side effect is rarely seen with Rybelsus.
Kidney problems: Rybelsus can cause what’s called acute kidney injury. This is seen more often in people who have a history of kidney problems and those who get dehydrated from vomiting.
Vision problems: Sometimes, lowering glucose levels too quickly can lead to a vision problem called diabetic retinopathy. This is more common if you had a history of vision issues before starting the medication.
Allergic reactions: Rybelsus can cause an allergic reaction or anaphylaxis, a severe allergy in some people. Signs of a serious allergic reaction include rash, chest tightness, wheezing or difficulty breathing, and flushing.
Rybelsus also carries a black box warning from the FDA about its potential to increase the risk of a type of thyroid cancer called medullary thyroid carcinoma.
If you’ve had this type of cancer or carry other risk factors for it, your provider won’t prescribe this drug to you. It’s important to note that this risk has only been seen in animal studies so far (FDA, 2017).
Rybelsus dosage
Rybelsus tablets are available in 3 mg, 7 mg, and 14 mg doses. This drug is only available with a prescription––there currently is no over-the-counter version.
The starting dose of Rybelsus is usually 3 mg once a day for 30 days, followed by 7 mg daily after that. Your healthcare provider may adjust the dosage based on your symptoms, side effects, other medications you’re taking, and how well you respond to the treatment.
Your provider will generally recommend taking Rybelsus in the morning, at least 30 minutes before eating or drinking anything. You should only take it with up to four ounces of plain water. If you take it with other drinks or food, the medication may be less effective. You should also take the tablets whole (meaning don’t cut them in half, grind them up, or chew them) (FDA, 2017).
Rybelsus warnings
People with certain underlying medical conditions have an increased risk of adverse effects from Rybelsus. It’s a good idea to consult your healthcare provider before taking Rybelsus, especially if you have any of the following risk factors (FDA, 2017):
History of medullary thyroid carcinoma
History of a disease called MEN2 (multiple endocrine neoplasia syndrome type 2) that puts you at risk for thyroid cancers
A past allergic reaction to any type of semaglutide medication
If you’ve had pancreatitis
If you have type 1 diabetes or diabetic ketoacidosis
If you’re pregnant or breastfeeding
Rybelsus drug interactions
Rybelsus can cause adverse reactions when combined with other medications. Before you take Rybelsus, let your healthcare provider know if you’re using any prescription drugs or supplements that may interact with it including (FDA, 2017):
Insulin: Both insulin and Rybelsus lower your blood sugar, so your blood sugar may get dangerously low if you take them together.
Metformin: Metformin is a type of drug called sulfonylurea, which lowers blood sugar. Your sugar levels may get too low if you mix metformin and Rybelsus.
Thyroid medication: Levothyroxine is a medication used to treat some thyroid conditions. If you take it with Rybelsus, it may become less effective. Rybelsus slows down how quickly your stomach empties, so it can also interfere with your body’s ability to get rid of levothyroxine, which increases your risk of side effects.
Rybelsus cost
Rybelsus is only available as a brand name drug and is not sold in a generic form. The average monthly price of Rybelsus is $1,011.72 (GoodRx, 2022).
Some pharmacies offer coupons to lower the cost. Many insurance companies also cover part or all of the bill; check with your insurance provider to get the best estimate of your monthly costs.
Sometimes it’s hard to get your blood sugar under control when living with type 2 diabetes, but medications like Rybelsus may be a good solution. A healthcare professional can help you decide whether this drug is the right treatment for you.
DISCLAIMER
If you have any medical questions or concerns, please talk to your healthcare provider. The articles on Health Guide are underpinned by peer-reviewed research and information drawn from medical societies and governmental agencies. However, they are not a substitute for professional medical advice, diagnosis, or treatment.
Anderson, S. L., Beutel, T. R., & Trujillo, J. M. (2020). Oral semaglutide in type 2 diabetes. Journal of Diabetes and its Complications , 34 (4), 107520. doi:10.1016/j.jdiacomp.2019.107520. Retrieved from https://pubmed.ncbi.nlm.nih.gov/31952996/
Davies, M., Pieber, T. R., Hartoft-Nielsen, M. L., Hansen, O., Jabbour, S., & Rosenstock, J. (2017). Effect of Oral Semaglutide Compared With Placebo and Subcutaneous Semaglutide on Glycemic Control in Patients With Type 2 Diabetes: A Randomized Clinical Trial. Journal of the American Medical Association , 318 (15), 1460–1470. doi:10.1001/jama.2017.14752. Retrieved from https://jamanetwork.com/journals/jama/fullarticle/2657376
GoodRx-a. (2022). Rybelsus . Retrieved Feb. 17, 2022 from https://www.goodrx.com/rybelsus
Granhall, C., Donsmark, M., Blicher, T. M., Golor, G., Søndergaard, F. L., Thomsen, M., et al. (2019). Safety and Pharmacokinetics of Single and Multiple Ascending Doses of the Novel Oral Human GLP-1 Analogue, Oral Semaglutide, in Healthy Subjects and Subjects with Type 2 Diabetes. Clinical Pharmacokinetics,
doi:10.1007/s40262-018-0728-4. Retrieved from https://link.springer.com/article/10.1007/s40262-018-0728-4
Husain, M., Bain, S. C., Holst, A. G., Mark, T., Rasmussen, S., & Lingvay, I. (2020). Effects of semaglutide on risk of cardiovascular events across a continuum of cardiovascular risk: combined post hoc analysis of the SUSTAIN and PIONEER trials. Cardiovascular Diabetology , 19 (1), 156. doi:10.1186/s12933-020-01106-4. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7526237/
Pratley, R., Amod, A., Hoff, S. T., Kadowaki, T., Lingvay, I., Nauck, M., et al. (2019). Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double-blind, phase 3a trial. Lancet (London, England), 394 (10192), 39–50. doi: 10.1016/S0140-6736(19)31271-1. Retrieved from https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(19)31271-1/fulltext
Shaefer, C. F., Jr, Kushner, P., & Aguilar, R. (2015). User's guide to mechanism of action and clinical use of GLP-1 receptor agonists. Postgraduate Medicine , 127 (8), 818–826. doi:10.1080/00325481.2015.1090295. Retrieved from https://pubmed.ncbi.nlm.nih.gov/26371721/
United States Food and Drug Administration (FDA). (2019). Highlights of Prescribing Information: Rybelsus. Retrieved on Feb. 7, 2022 from https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/213051s000lbl.pdf










