Key takeaways
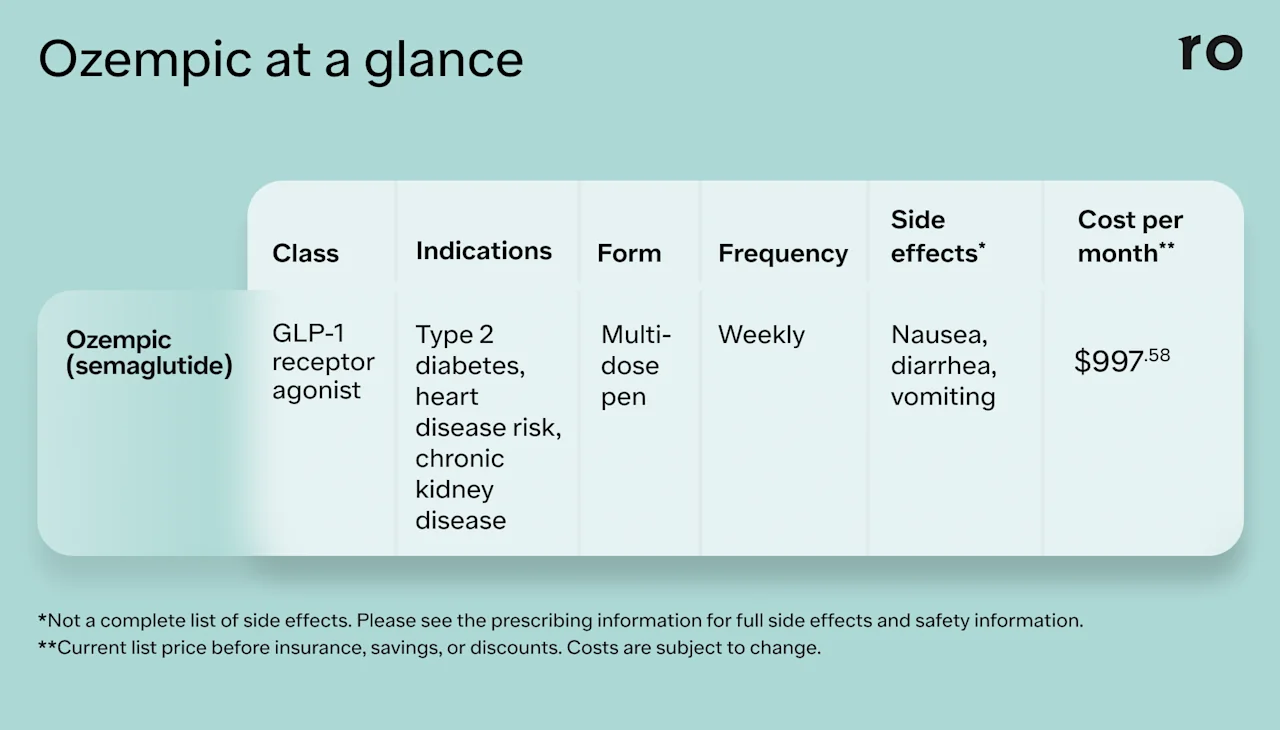
Ozempic (semaglutide) is an injectable GLP-1 drug. It is approved to treat type 2 diabetes and to reduce the risk of heart disease and kidney failure in certain populations. It may be prescribed off-label for weight loss.
Ozempic can help promote weight loss by mimicking hormones that help to reduce hunger and increase feelings of fullness after eating.
Common side effects include gastrointestinal(GI)complaints like nausea, diarrhea, and constipation. Side effects tend to be mild and go away over time.
Here's what we'll cover
Here's what we'll cover
Key takeaways
Ozempic (semaglutide) is an injectable GLP-1 drug. It is approved to treat type 2 diabetes and to reduce the risk of heart disease and kidney failure in certain populations. It may be prescribed off-label for weight loss.
Ozempic can help promote weight loss by mimicking hormones that help to reduce hunger and increase feelings of fullness after eating.
Common side effects include gastrointestinal(GI)complaints like nausea, diarrhea, and constipation. Side effects tend to be mild and go away over time.
By now, Ozempic (semaglutide) has become a household name—this breakthrough drug went viral online in 2021, and its popularity has only grown since then. But what is Ozempic, and what’s all the buzz about?
Ozempic belongs to a drug class called glucagon-like peptide-1 receptor agonists, or GLP-1s. While it was originally developed to treat type 2 diabetes, researchers eventually discovered that it can also be highly effective for weight loss. As a result, Ozempic and other GLP-1s have transformed the way we think about obesity treatment.
If you’re wondering whether Ozempic might be a good weight loss option for you, this article can help. Keep reading to understand what Ozempic is, how it works, common side effects, and how much weight you could lose while taking it.
What is Ozempic?
Ozempic (semaglutide) belongs to a class of drugs called GLP-1 receptor agonists.
The Ozempic pen is a multi-dose pen that contains enough medication for one month. Each week, you attach a new needle to the pen, inject the medicine, and then remove and dispose of the needle.
Ozempic is injected under the skin (subcutaneously) of the belly, thigh, or upper arm once a week. The injection pens come in 0.25 mg, 0.5 mg, 1 mg, and 2 mg strengths. The starting dose is typically 0.25 mg weekly for four weeks. After that, your healthcare provider may increase your dose to 0.5 mg once a week.
Some people may stay at 0.5 mg as a maintenance dose. If you need additional blood sugar control, your healthcare provider may increase the dose every four weeks. The maximum dose of Ozempic is 2 mg weekly.
What is Ozempic used for?
Ozempic was approved by the US Food and Drug Administration (FDA) to help regulate blood glucose (sugar) in people with type 2 diabetes. It also helps to reduce the risk of major heart events like heart attack and stroke in adults with type 2 diabetes and cardiovascular disease.
In January 2025, the FDA approved Ozempic for a third indication: to reduce the risk of worsening kidney disease, kidney failure, and cardiovascular disease-related death in adults with type 2 diabetes and chronic kidney disease.
Some healthcare providers may also prescribe Ozempic off-label (for a condition it wasn't approved to treat) to help promote weight loss in people with obesity or overweight. In 2021, semaglutide (the active ingredient in Ozempic) was also approved for weight loss under the brand name Wegovy.
What does Ozempic do?
Ozempic helps lower blood sugar by helping your pancreas release more insulin—a hormone that regulates blood sugar levels. It also acts on hunger cues in the brain to reduce appetite and increase feelings of fullness after eating, which can lead to weight loss.
By regulating blood sugar and promoting weight loss, Ozempic may also reduce cardiovascular risks.
"Obesity is one of the biggest risk factors for cardiac issues," says Raj Singh, MD, FACP, FASN, an internal medicine specialist, nephrologist, and obesity medicine specialist. "Most of Ozempic's effects are related to causing weight loss. Once they lose weight, people get healthier, their diabetes gets better, and their cholesterol gets better."
These effects on blood sugar control, weight, and cardiovascular health may also have a protective effect on kidney function in people with existing kidney disease. Researchers believe that semaglutide may help reduce inflammation, improve blood flow, and reduce oxidative stress in the kidneys.
How does Ozempic work?
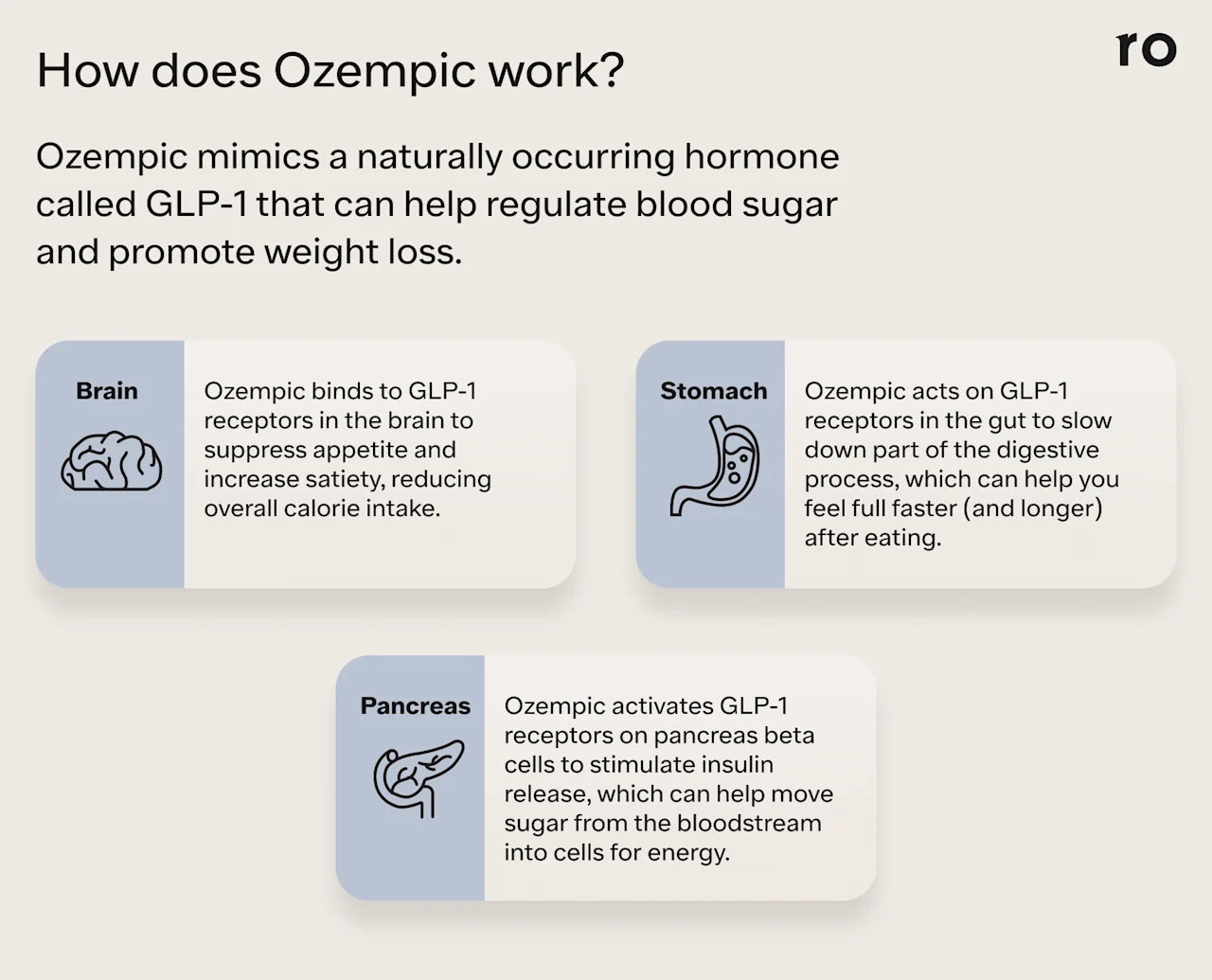
Ozempic works by mimicking a natural hormone called GLP-1 in the pancreas, stomach, and brain. GLP-1 plays a role in regulating appetite, blood sugar, and satiety (how satisfied you feel after eating). After you eat, levels of this gut hormone rise. GLP-1 acts on receptors in your brain to dampen feelings of hunger, which can reduce appetite and how many calories you take in. "It signals to the body that you're full," says Dr. Singh.
GLP-1 also stimulates the release of the hormone insulin from your pancreas. Insulin helps to move glucose (sugar) from your bloodstream into your cells, which can lower your blood sugar. GLP-1 also reduces the release of glucagon, another hormone that raises your blood sugar. The combined effect of these two actions is to improve blood sugar control.
Another weight loss-promoting action of Ozempic is to slow gastric emptying, or how quickly food moves from your stomach to your lower intestine. Keeping food in your stomach helps you stay full for longer, which may reduce the amount of food you eat and stop food noise and cravings.
Ozempic for weight loss: does it work?
In short, yes. When combined with regular exercise and a balanced diet, studies show that Ozempic can be an effective tool for weight loss.
Most clinical trials that measure weight loss with semaglutide involve people taking 2.4 mg weekly, which is the maximum dose of Wegovy (the maximum weekly dose of Ozempic is 2 mg), though some studies evaluate weight loss at lower dose strengths. While Ozempic hasn’t been studied specifically for its impact on weight, available research on semaglutide can help paint a picture of the weight loss potential with Ozempic.
Here’s what the research says:
One review of semaglutide versus placebo (inactive treatment) looked at four randomized-controlled trials. The studies, which were conducted between 2018 and 2021, included 3,613 total participants with obesity or overweight plus at least one health-related health condition. People who were treated with semaglutide lost almost 12% of their body weight compared with the placebo group.
The higher the dose of semaglutide, the more significant the weight loss. Another review of 23 randomized controlled trials (a type of study design deemed the gold standard in clinical trials) included 11,545 people with overweight or obesity, with or without type 2 diabetes, who took semaglutide, liraglutide, or other blood sugar-lowering drugs. People who took the 2.4 mg dose of semaglutide lost the most weight—more than 27 pounds. Their hemoglobin A1C levels, a marker of blood sugar control, also decreased.
In one early study, people with type 2 diabetes who took 1 mg of semaglutide each week lost an average of 10 pounds after 30 weeks on the medication. They also saw significant decreases in A1c levels.
In other clinical trials, people have lost as much as 15% of their original weight within 68 weeks (a little over a year) on semaglutide. They also saw improvements in cardiovascular risks and overall health, according to a 2023 review of studies on semaglutide for weight loss.
It's important to note that weight loss results are very individual. The amount of weight you can lose on Ozempic (or any other weight loss injection) varies. Incorporating healthy lifestyle habits like a balanced diet and regular exercise can help maximize weight loss and promote better health overall. Dr. Singh recommends eating complex carbohydrates like fruits and vegetables, beans, and whole grains, plus getting extra protein to prevent possible muscle loss while you take Ozempic.
Dr. Singh also points out that GLP-1 drugs aren't meant to be a quick weight loss solution. They're intended for long-term use. If you stop taking Ozempic, your food cravings may return, and you may also regain some or all of the weight you lost.
Potential side effects and risks
Ozempic went through an extensive clinical review and approval process and is considered a safe medication.
The most common side effects of Ozempic are mainly gastrointestinal (GI). These side effects may be more noticeable when you start taking Ozempic or when you increase the dose, but they tend to go away over time.
Here are some of the most common Ozempic side effects and tips to manage them:
Nausea. Eat bland foods like crackers about an hour after you take the medicine. You can also try ginger, which can help with nausea. Avoid fatty and spicy foods. Eat several small meals a day instead of three big ones.
Vomiting. Stay hydrated by drinking extra water or electrolyte drinks. Ask your healthcare provider about prescription or over-the-counter medications to help with vomiting.
Diarrhea. Avoid high-fiber foods and dairy until your symptoms improve. Drink extra fluids to stay hydrated.
Constipation. Add more fiber to your diet from foods like fruits and vegetables, beans, and whole grains.
Warnings and precautions
Ozempic also comes with more serious risks and precautions, though these are rare. Regular monitoring by your healthcare provider can help minimize these risks.
Possible rare but serious side effects include:
Inflammation of the pancreas (pancreatitis)
Complications of the eye disease diabetic retinopathy in people with type 2 diabetes
Gallbladder disease
Low blood sugar (hypoglycemia)
Lung aspiration (inhaling food or fluids into the lungs) during general anesthesia or deep sedation for a medical procedure
Severe allergic reactions like anaphylaxis
Kidney injury
Gallstones or inflammation of the gallbladder
Ozempic has a black box warning about an increased risk for thyroid C-cell tumors. In studies, this medication caused thyroid tumors in rats. Even though human studies have not shown the same effect, anyone with a personal or family history of multiple endocrine neoplasia syndrome type 2 (MEN 2), a rare cancer that increases the risk for thyroid tumors, should not take Ozempic.
Ozempic may interact with other medications, especially ones prescribed to treat diabetes since it stimulates the release of insulin, which lowers blood sugar. Taking Ozempic with insulin or other medicines that increase insulin could lead to very low blood sugar called hypoglycemia. Examples of these medicines, called sulfonylurea drugs, are:
Chlorpropamide (Diabinese)
Glyburide (DiaBeta, Glynase)
Glipizide (Glucotrol, Glucotrol XL)
Glimepiride (Amaryl)
Tell your healthcare provider if you are taking these or any other sulfonylurea drugs before you start on Ozempic.
Who should not take Ozempic?
Although Ozempic is generally considered safe, it may not be an appropriate option for everyone. You should not take Ozempic if you:
Are under the age of 18
Are pregnant, breastfeeding, or planning to become pregnant
Don't have a healthcare provider’s prescription
Have kidney disease, liver disease, or pancreatitis
Have a known allergy to semaglutide
Have a personal or family history of thyroid cancer
Comparing Ozempic to other weight loss medications
Ozempic isn't the only medication on the market that can promote weight loss. Several GLP-1s are specifically designed and approved for weight loss and might be good alternatives to consider.
Wegovy
Wegovy contains the same active ingredient as Ozempic, semaglutide. Unlike Ozempic, it's approved for chronic weight management in adults ages 18 and older with either obesity, or overweight plus at least one weight-related condition like type 2 diabetes, high blood pressure, or high cholesterol. Wegovy is also approved to help reduce the risk of major cardiovascular events (such as heart attack and stroke) in people with obesity and overweight who also have heart disease.
Because they share the same active ingredient, both drugs are similar and basically work in the same way, but Wegovy and Ozempic have some key differences. Wegovy comes in different dose strengths, with a maximum dose of 2.4 mg. Wegovy may also lead to more weight loss than Ozempic—one study found that people who took 2.4 mg of semaglutide lost about 15% of their body weight after 68 weeks (about a year and three months).
Saxenda
Saxenda (liraglutide) is an injectable GLP-1 receptor agonist that you take daily instead of weekly. It’s approved for weight loss in adults with obesity or people with overweight who have a related health condition like high blood pressure or type 2 diabetes. It’s also approved for weight management in children aged 12 and older who have obesity.
In one clinical trial, people who took the highest dose of Saxenda (3 mg) lost around 5-10% of their body weight after about a year on the medication.
Zepbound
Zepbound (tirzepatide) is a slightly different type of weight-loss drug. It is a dual-action receptor agonist that works on both GLP-1 and gastric inhibitory polypeptide (GIP). GIP is a hormone released by the gut that stimulates the release of insulin to regulate blood sugar levels and metabolism (how our bodies burn food for energy).
Some research shows that tirzepatide may help people lose more of their body weight (15% or more of their starting weight) compared with semaglutide. "It has a dual action," Dr. Singh says."That's why newer medications like Zepbound are more effective at [promoting] weight loss than semaglutide."
Ozempic cost and insurance coverage
Ozempic costs an average of $997.58 per month without insurance. Manufacturer savings cards, coupons, or direct-to-patient programs may help reduce out-of-pocket costs. For example, Novo Nordisk (the maker of Ozempic) offers a savings card where eligible patients with commercial or private insurance could pay just $25 for a one- to three-month supply of Ozempic for up to two years.
While most insurance companies will cover the cost of this medication when it's used to treat type 2 diabetes, it usually isn't covered when used off-label for weight loss. Some people choose to cover the cost themselves. If you’re paying out of pocket, you may be able to find Ozempic coupons from popular drug discount sites.
How to inject and store Ozempic
It can take some time and practice to learn how to inject Ozempic. Dr. Singh says that your provider may do the first injection in the office to show you how to select the correct dose, clean the site, give yourself the injection, and store the medication.
Ozempic comes in an auto-injector pen. The manufacturer recommends storing the pens in the refrigerator at 36℉–46℉.
Once you’ve gathered your supplies and thoroughly washed your hands, follow these steps to administer Ozempic:
Check that the liquid inside the pen isn't cloudy.
Put a new needle onto the auto-injector pen with each use. To attach the needle, push it onto the pen and then turn it until it's on tight.
Remove the outer cap and the inner cap from the needle.
Select the correct dose by turning the dose selector.
Choose an injection site on the skin of your stomach, thigh, or upper arm. Rotate the site each time you inject.
Clean the skin.
Insert the needle and press the button until the dose counter shows 0.
Remove the needle and dispose of it in a sharps container or a hard plastic bottle.
Myths and misconceptions about Ozempic
Ozempic has gotten a lot of media coverage because it can produce such substantial weight loss. But amid all the buzz, misconceptions about the drug have spread.
Myth: Ozempic is a quick fix for weight loss
First, Ozempic isn't approved for weight loss. It's a type 2 diabetes drug that helps to lower blood sugar—weight loss is just a secondary benefit of the medication. And while this drug might help you lose a significant amount of weight, Ozempic isn't magic. "There is no such thing as a 'quick fix,'" Dr. Singh stresses.
While Ozempic can be helpful for people who have struggled for many years to lose weight, maintaining weight loss and blood sugar control requires that you also make lifestyle changes and stick with the medication long-term. Eating a healthy diet and getting regular exercise may also help reduce rebound weight gain if you do eventually stop taking this medication.
Myth: Anyone can use Ozempic without a prescription
Ozempic is only available with a prescription from a licensed healthcare provider. Given the possible risks, it’s important to take Ozempic safely and under the care of a knowledgeable healthcare provider.
"These medications have side effects, and people should be properly evaluated to make sure they are healthy enough to lose weight before they start on them," Dr. Singh says.
How to get started with Ozempic
Talk to your healthcare provider if you’re interested in taking Ozempic for weight loss. They can do a complete workup and evaluate your personal and family health history to determine if you’re a good candidate for Ozempic, or if another treatment might be better suited to your health needs and goals. They may recommend a different medication that’s specifically indicated for weight loss, such as Wegovy, Zepbound, or Saxenda.
They should discuss with you not only whether you're eligible to take this medicine, but also how much you can expect to pay and whether your health insurance will cover any of the cost.
Online weight loss programs such as Ro’s Body membership may be another option. Ro’s membership connects you with a licensed healthcare provider who can provide a personalized health plan that includes coaching, support, and if appropriate, medications like Ozempic or other GLP-1s.
Once you start taking a GLP-1, regular medical monitoring is key to ensuring that you're on the correct dose, and you're meeting your goals. "Generally, it will be about a monthly check-in to see how things are and what kinds of side effects you're experiencing," Dr. Singh says. He says that blood tests may be taken about once every three months to check that your blood sugar levels are improving.
"If you are experiencing side effects, then generally we titrate the dose down a little bit so your body can get used to the medication," he adds. Your healthcare provider can also recommend medication and lifestyle changes to manage any side effects you are experiencing. If you're responding to the drug but not meeting your A1C goals or not losing weight on Ozempic, your provider might increase the dose.
Bottom line
Ozempic can be an effective treatment for type 2 diabetes that can also help with weight loss. If you’re curious about getting Ozempic for weight loss, talk to a healthcare provider, such as your primary care physician or one through Ro. They can evaluate your personal and family health history, overall health, and other factors to help you determine whether Ozempic is an appropriate way to help you meet your blood sugar and weight loss goals.
Ozempic (semaglutide) is FDA-approved to treat type 2 diabetes and to reduce the risk of major cardiac events and chronic kidney disease in certain populations. In some cases, healthcare providers may prescribe it off-label for weight loss.
Ozempic mimics the GLP-1 hormone, activating its receptors to improve blood sugar control, help reduce appetite, slow digestion, and increase feelings of fullness. The latter effects, in particular, can play key roles in promoting weight loss.
The most common side effects are GI issues like nausea, vomiting, and constipation. These side effects tend to be mild and should improve once you've been on the medication for a while.
This drug is meant to be taken long-term. Stopping it too soon may cause rebound weight gain or compromise your blood sugar control.
Ozempic can cost around $1000 each month. Though it's more likely to be covered if you’re taking it for type 2 diabetes, not every insurance provider will cover the cost. Savings programs, coupons, and other discounts may help make this medicine more affordable.
Frequently asked questions (FAQs)
What does Ozempic do for weight loss?
Ozempic acts like a hormone your body makes called GLP-1. It helps you feel full sooner after eating, reduces hunger cues in the brain, and helps to control your blood sugar levels.
How much weight do you lose in a month with Ozempic?
The amount of weight loss with Ozempic varies from person to person. Given that this drug is designed to treat type 2 diabetes (not help with weight loss) and that it's meant for long-term use, it can be hard to pinpoint the exact amount of weight you can expect to lose on it in a month.
That said, research may offer some cues as to the amount of weight a person could potentially lose with Ozempic. In one study, researchers followed 408 people who had a body mass index (BMI) of 27 or more for three months as they received weekly injections of 1.7 mg or 2.4 mg of semaglutide (the maximum dose of Wegovy). The average weight loss at three months was about 15 pounds, which works out to about 5 pounds lost per month. Keep in mind that weight loss is individual and may be impacted by factors like age, starting weight, physical activity, overall health, and more.
Is Ozempic approved for weight loss?
No. Ozempic is approved for the treatment of type 2 diabetes as well as other non-weight-loss-specific indications. But it's sometimes prescribed off-label for weight loss.
What happens if you take Ozempic without diabetes?
You can take Ozempic if you don't have diabetes. The medication comes with the same potential side effects and warnings whether you take it for diabetes or weight management. But because Ozempic isn’t FDA-approved for weight loss, insurance generally won't cover the cost, and you may have to pay for the drug out of pocket.
There are also concerns about potential drug shortages with off-label use of diabetes drugs for weight loss, which can make it harder for people with type 2 diabetes to access Ozempic.
What are the downsides of Ozempic?
Ozempic can cause side effects. The most common ones are mild GI issues like nausea, vomiting, diarrhea, and constipation. These common side effects tend to be mild and manageable and go away the longer you’re on the medication. Rarely, more serious problems like pancreatitis, kidney damage, gallbladder disease, and severe allergic reactions have occurred with this medication.
Another potential downside is cost—Ozempic can cost up to about $1,000 per month without insurance, savings programs, and other discounts. Insurance plans typically do not cover off-label medications, so you may have to pay out of pocket.
DISCLAIMER
If you have any medical questions or concerns, please talk to your healthcare provider. The articles on Health Guide are underpinned by peer-reviewed research and information drawn from medical societies and governmental agencies. However, they are not a substitute for professional medical advice, diagnosis, or treatment.
Ozempic Important Safety Information: Read more about serious warnings and safety info.
GLP-1 Important Safety Information: Read more about serious warnings and safety info.
Wegovy Important Safety Information: Read more about serious warnings and safety info.
Saxenda Important Safety Information: Read more about serious warnings and safety info.
Zepbound Important Safety Information: Read more about serious warnings and safety info.
Chao, A. M., Tronieri, J. S., Amaro, A., et al. (2021) Semaglutide for the treatment of obesity. Trends in Cardiovascular Medicine, 33(3), 159-166. doi: 10.1016/j.tcm.2021.12.008. Retrieved from https://pmc.ncbi.nlm.nih.gov/articles/PMC9209591/
Ghusn, W., De la Rosa, A., Sacoto, D., et al. (2022). Weight loss outcomes associated with semaglutide treatment for patients with overweight or obesity. JAMA Network Open, 5(9), e2231982. doi: 10.1001/jamanetworkopen.2022.31982. Retrieved from https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2796491
Han, S. H., Safeek, R., Ockerman, K., et al. (2023). Public Interest in the Off-Label Use of Glucagon-like Peptide 1 Agonists (Ozempic) for Cosmetic Weight Loss: A Google Trends Analysis. Aesthetic Surgery Journal, 44(1), 60-67. doi: 10.1093/asj/sjad211. Retrieved from https://pubmed.ncbi.nlm.nih.gov/37402640/
Hinnen, D. (2017). Glucagon-like peptide 1 receptor agonists for type 2 diabetes. Diabetes Spectrum, 30(2), 202-210. doi: 10.2337/ds16-0026. Retrieved from https://pmc.ncbi.nlm.nih.gov/articles/PMC5556578/
NAIC. (2024). Does insurance cover prescription weight-loss injectables? Retrieved from https://content.naic.org/article/does-insurance-cover-prescription-weight-loss-injectables
Novo Nordisk. (2024). How to use the Ozempic pen. Retrieved from https://www.ozempic.com/how-to-take/ozempic-pen.html
Novo Nordisk. (2023). Ozempic pricing. Retrieved from https://www.novopricing.com/ozempic.html
Pi-Sunyer, X., Astrup, A., Fujioka, K., et al. (2015). A Randomized, Controlled Trial of 3.0 mg of Liraglutide in Weight Management. The New England Journal of Medicine, 373(1), 11–22. doi: 10.1056/NEJMoa1411892. Retrieved from https://pubmed.ncbi.nlm.nih.gov/26132939/
Rodriguez, P. J., Cartwright, B. M. G., Gratzl, S., et al. (2024). Semaglutide vs. tirzepatide or weight loss in adults with overweight or obesity. JAMA Internal Medicine, 184(9), 1056-1064. doi: 10.1001/jamainternmed.2024.2525. Retrieved from https://pmc.ncbi.nlm.nih.gov/articles/PMC11231910/
Shaman, A. M., Bain, S. C., Bakris, G. L., et al. (2022). Effect of the Glucagon-Like Peptide-1 Receptor Agonists Semaglutide and Liraglutide on Kidney Outcomes in Patients With Type 2 Diabetes: Pooled Analysis of SUSTAIN 6 and LEADER. Circulation, 145(8), 575–585. doi: 10.1161/CIRCULATIONAHA.121.055459, Retrieved from https://pmc.ncbi.nlm.nih.gov/articles/PMC8860212/
Sorli, C., Harashima, S. I., Tsoukas, G. M., et al. (2017). Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. The Lancet. Diabetes & Endocrinology, 5(4), 251–260. doi: 0.1016/S2213-8587(17)30013-X. Retrieved from https://pubmed.ncbi.nlm.nih.gov/28110911/
Tan, H. C., Dampil, O. A., & Marquez, M. M. (2022). Efficacy and safety of semaglutide for weight loss in obesity without diabetes: A systematic review and meta-analysis. Journal of the ASEAN Federation of Endocrine Societies, 37(2), 65-72.doi: 10.15605/jafes.037.02. Retrieved from https://pmc.ncbi.nlm.nih.gov/articles/PMC9758543/
U.S. Food and Drug Administration (FDA). (2024). Ozempic semaglutide Injection. Retrieved from https://www.novo-pi.com/ozempic.pdf
Xie Z., Yang, S., Deng, W. et al. (2022). Efficacy and Safety of Liraglutide and Semaglutide on Weight Loss in People with Obesity or Overweight: A Systematic Review. Clinical Epidemiology, 14, 1463-1476. doi: 10.2147/CLEP.S391819. Retrieved from https://pmc.ncbi.nlm.nih.gov/articles/PMC9738168/