Key takeaways
To qualify for semaglutide, you need a prescription from a licensed healthcare provider.
You may qualify with a BMI of 30 or higher, or a BMI of 27 or higher with a weight-related condition such as high blood pressure, diabetes, or high cholesterol.
Eligibility varies by brand: Ozempic and Rybelsus are approved for type 2 diabetes, while Wegovy is approved for weight loss and fatty liver disease.
Here's what we'll cover
Here's what we'll cover
Here's what we'll cover
Key takeaways
To qualify for semaglutide, you need a prescription from a licensed healthcare provider.
You may qualify with a BMI of 30 or higher, or a BMI of 27 or higher with a weight-related condition such as high blood pressure, diabetes, or high cholesterol.
Eligibility varies by brand: Ozempic and Rybelsus are approved for type 2 diabetes, while Wegovy is approved for weight loss and fatty liver disease.
Semaglutide, the active ingredient in brand-name medications like Ozempic, Wegovy, and Rybelsus, has been shown to support significant weight reduction in certain patients.
To qualify for semaglutide, you’ll typically need to meet specific criteria: either a body mass index (BMI) of 30 or higher, or a BMI of 27 or higher with at least one weight-related health condition such as high blood pressure, type 2 diabetes, or high cholesterol. A licensed healthcare provider will ultimately determine if semaglutide is appropriate for you.
In this article, we’ll walk through who may qualify for semaglutide, how to get a prescription (both in person and online, including Ro’s weight loss program, and what to know about costs and insurance coverage before starting treatment.
How to qualify for semaglutide
If you’re wondering how you can qualify for semaglutide, know it’s not always super straightforward. Qualification for semaglutide depends on the specific drug and its uses or indications approved by the US Food and Drug Administration (FDA).
Semaglutide is the active ingredient in the following brand-name drugs: Ozempic, Wegovy, and Rybelsus. While all three are part of a class of drugs known as GLP-1s (short for glucagon-like peptide 1 receptor agonist), they have different indications and, therefore, different eligibility requirements.
We will break down the details for each version of semaglutide.
Who qualifies for Ozempic?
Ozempic is an FDA-approved medication for adults with type 2 diabetes. It works to improve blood sugar control and offers additional benefits, such as reducing the risk of major cardiovascular events (like heart attack and stroke) and lowering the risk of kidney complications in those with type 2 diabetes and chronic kidney disease.
While Ozempic is not FDA-approved for weight loss, healthcare providers sometimes prescribe it off-label for chronic weight management. “Off-label” means a medication is used for a purpose other than its official FDA approval.
If you’re considering Ozempic for weight management, eligibility is determined by your provider since it is prescribed off-label. They may look at factors like:
Body mass index (BMI): Generally, a BMI of 30 or higher is considered obesity, and 25 or higher is considered overweight. You can calculate your BMI using this calculator, but keep in mind this is not a hard and fast rule and your BMI may not determine whether you qualify.
Weight-related health conditions: These may include high blood pressure, type 2 diabetes, or high cholesterol.
In many cases, providers apply criteria similar to those used for Wegovy (another semaglutide medication FDA-approved for weight management).
Ultimately, a licensed provider will determine if Ozempic is appropriate for you.
Who qualifies for Wegovy?
Wegovy is a once-weekly injection that also contains semaglutide. Unlike Ozempic, which is FDA-approved for type 2 diabetes, Wegovy is FDA-approved specifically for chronic weight management when combined with a reduced-calorie diet and increased physical activity.
To qualify for Wegovy, you’ll typically need one of the following:
A BMI of 30 or higher (classified as obesity), or
A BMI of 27 or higher with at least one weight-related health condition
Weight-related conditions may include:
High blood pressure (hypertension)
High cholesterol (dyslipidemia)
Type 2 diabetes
Heart disease
Obstructive sleep apnea
Polycystic ovary syndrome (PCOS)
In addition, in March 2024 the FDA expanded Wegovy’s approval to include reducing the risk of major cardiovascular events — like heart attack, stroke, and cardiovascular death — in adults with cardiovascular disease plus overweight or obesity.
In August 2025, the FDA approved Wegovy for another use: to treat a type of fatty liver disease called metabolic dysfunction-associated steatohepatitis (MASH) in adults who have moderate to advanced liver scarring (fibrosis) but do not have permanent liver damage (cirrhosis). MASH, aka nonalcoholic steatohepatitis, results from metabolism problems that lead to excess fat buildup and inflammation in the liver.
For all of Wegovy’s approved uses, the medication is meant to be combined with a reduced-calorie diet and increased physical activity.
Who qualifies for Rybelsus?
Unlike Ozempic and Wegovy, which are injectables, Rybelsus comes in pill form. For the needle-phobic out there, this might sound like a dream. But here’s the catch: Rybelsus is currently only approved, alongside diet and exercise, to improve glycemic control in individuals with type 2 diabetes.
Your provider may prescribe Rybeusus off-label for weight loss, similarly to Ozempic, but it is much more rare. That’s because the dosing of the oral medication makes it relatively ineffective for weight loss.
Eligibility is often similar to Wegovy, meaning you’ll typically need a BMI above a specific cutoff or a health condition linked to weight, like high blood pressure or type 2 diabetes.
Who qualifies for compounded semaglutide?
Because compounded semaglutide isn’t FDA-approved, there are no standardized guidelines or official indications for who qualifies for it. Compounded medications do not undergo FDA review for safety, effectiveness, or manufacturing. They may be prescribed in situations where a patient’s needs can’t be met with a commercially available drug or when there’s a shortage of approved medications.
Instead, eligibility is typically determined by individual healthcare providers, often based on the same criteria used for FDA-approved versions like Ozempic or Wegovy (e.g. type 2 diabetes, obesity or overweight, etc.).
How long can you stay on semaglutide?
There’s no set time limit on how long you can take semaglutide. As long as you tolerate the medication well and your healthcare provider determines it’s the right option for you, semaglutide can be used long-term — sometimes even lifelong.
Since semaglutide was originally developed to help people with type 2 diabetes manage blood sugar, it’s designed for ongoing use as a treatment for chronic health conditions. The same applies when it’s prescribed for weight management.
Studies show that weekly semaglutide injections can be safe and effective for extended periods. Research also found that people who stopped semaglutide regained about two-thirds of the weight they had lost within a year. The takeaway? Obesity (and other conditions treated with semaglutide) are chronic, so long-term treatment may be needed to maintain long-term benefits.
That said, staying on semaglutide is a decision you should make with your provider based on your health goals, how you respond to treatment, and whether the benefits continue to outweigh any side effects.
Do you need a prescription for semaglutide for weight loss?
One word? Yes.
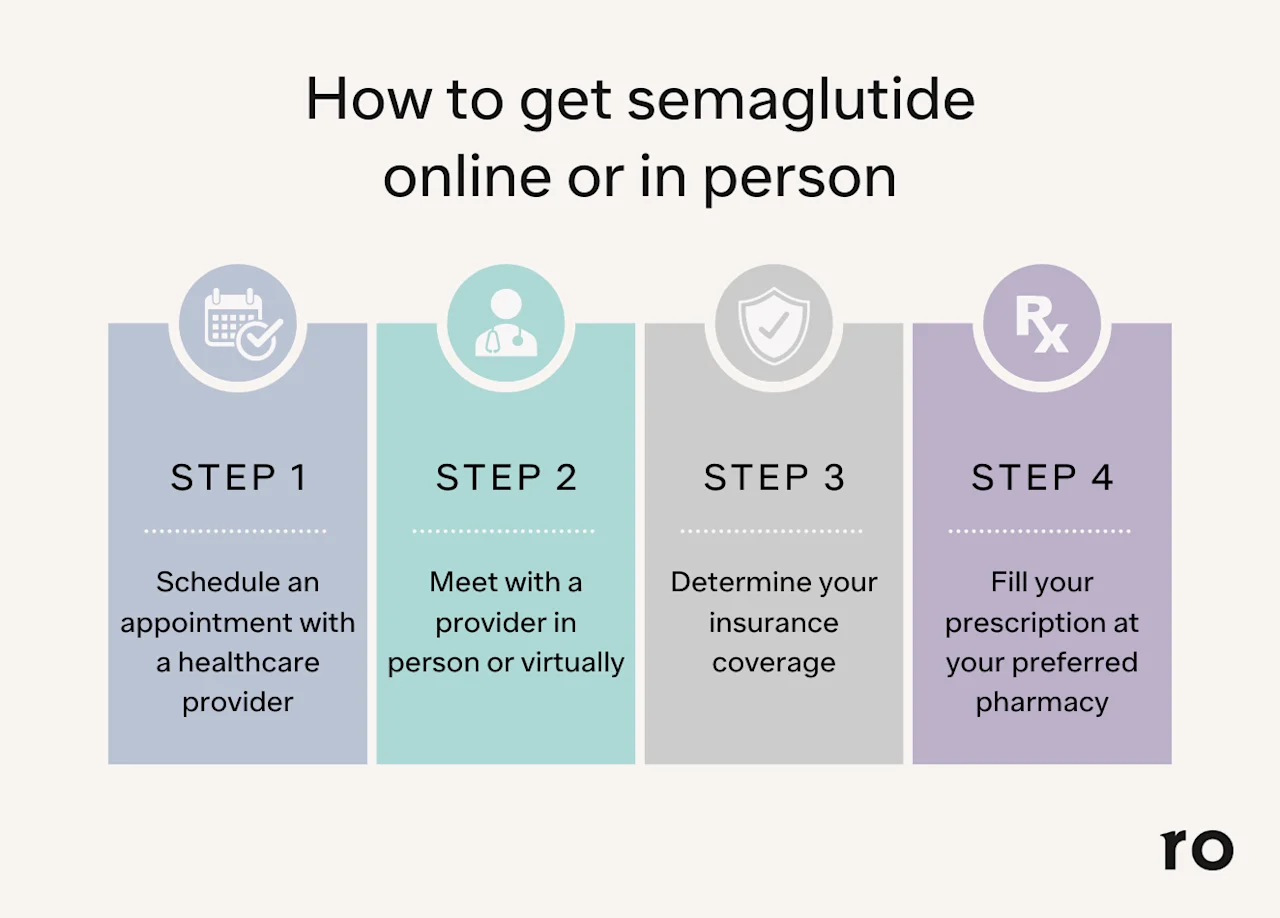
To get semaglutide for weight loss or another purpose, you will need a prescription from a licensed healthcare provider. Providers who can prescribe semaglutide include:
Doctor of Medicine (MD) or Doctor of Osteopathic Medicine (DO), such as your primary care provider (PCP), an endocrinologist, or another provider who specializes in weight loss
Nurse practitioner (NP)
Physician assistant (PA)
Semaglutide is generally considered safe for most people. Still, like any prescription medication, it’s not necessarily a fit for everyone — plus, it can interact with other drugs and comes with the potential for side effects. For these reasons (and because, you know, it’s legally required), it’s important to get semaglutide from a reputable healthcare provider that can make sure you are a good candidate for the drug and can monitor you for any potential issues.
You can talk to a licensed provider at Ro who can help you determine whether you qualify for semaglutide and potentially help you get insurance coverage for semaglutide. You can start to find out if you qualify here.
Who shouldn’t take semaglutide for weight loss?
Semaglutide is considered safe for many people who meet the medical and weight-related criteria, but it’s not right for everyone. You should avoid taking semaglutide if:
You are pregnant, planning to become pregnant soon, or breastfeeding
You have a personal or family history of medullary thyroid carcinoma (a rare type of thyroid cancer). All GLP-1 medications (including semaglutide) have been shown to cause thyroid tumors in rodents. More research is needed to better understand the overall risk, if any, to humans
You have multiple endocrine neoplasia syndrome type 2 (MEN2), a rare inherited disorder that causes tumors
You’ve had pancreatitis in the last six months
You are allergic to semaglutide or any of its ingredients
You are planning to have surgery or other procedures that use anesthesia
Stop taking semaglutide and seek immediate medical care if you develop symptoms such as:
Trouble breathing or swallowing
Swelling of the tongue or lips
Severe itching or hives
Rapid heartbeat, dizziness, or fainting
Thoughts of harming yourself
Other important considerations Before starting semaglutide, tell your healthcare provider if you have or have had:
Kidney or pancreas problems
Type 2 diabetes with a history of diabetic retinopathy
Depression, suicidal thoughts, or other mental health concerns
You should also let your provider know about any medications you take (including prescriptions, over-the-counter drugs, vitamins, or supplements).
With this information, your provider can help determine whether semaglutide is a safe and appropriate option for you.
Will insurance cover semaglutide?
Insurance coverage for semaglutide depends on several factors, including:
Which brand-name drug you’re prescribed (Ozempic, Wegovy, or Rybelsus)
Whether it’s being prescribed for type 2 diabetes, weight management, or another health condition
The specific rules of your insurance plan
When you may struggle to get covered:
Some insurance carriers do not cover weight loss medications at all
Others may require strict qualification criteria or prior authorization (extra paperwork from your healthcare provider proving medical necessity)
Coverage may differ by brand — for example, Ozempic is often easier to get covered if you have diabetes, while Wegovy may be harder since it’s approved specifically for weight management and fatty liver disease
Medicare and Medicaid:
Medicare does not cover semaglutide for weight loss. It may cover it if prescribed for type 2 diabetes, heart disease, kidney disease, or fatty liver disease.
Medicaid coverage varies by state. Some plans cover Wegovy for weight loss or fatty liver disease, while others do not.
Costs without insurance:
Because there is no generic semaglutide available, out-of-pocket costs can be high. According to Novo Nordisk (the drug manufacturer), list prices are:
The actual amount you pay may be lower depending on your insurance, pharmacy choice, location, or if you use discount programs, coupons, or manufacturer savings cards.
If your plan doesn’t cover semaglutide, talk with your provider about alternative medications or programs that may help make treatment more affordable.
Ro can also help you find out if you have insurance coverage by using our free insurance checker.
How to qualify for semaglutide: bottom line
Semaglutide can be an effective tool for managing weight, type 2 diabetes, and even reducing certain cardiovascular and kidney or liver risks—but it isn’t for everyone.
To qualify for semaglutide, here’s what you need to know:
There will always be a prescription required: You must qualify through a licensed healthcare provider, who will decide if semaglutide is right for you.
What you need will vary by medication: To qualify for Ozempic or Rybelsus, you typically need a diagnosis of type 2 diabetes. To qualify for Wegovy for weight loss, you’ll typically need a BMI of 30 or higher (obesity) or a BMI of 27 or higher (overweight) with a weight-related health condition.
Semaglutide is typically taken with lifestyle changes: Even if you qualify, semaglutide works best when paired with healthy eating and physical activity.
Insurance coverage will depend on many factors: Whether you qualify for insurance coverage depends on your plan, the specific brand prescribed, and the medical reason for treatment.
If you don’t qualify for semaglutide, don’t be discouraged. Other weight loss medications and treatment options may be a better fit for your situation and health goals.
DISCLAIMER
If you have any medical questions or concerns, please talk to your healthcare provider. The articles on Health Guide are underpinned by peer-reviewed research and information drawn from medical societies and governmental agencies. However, they are not a substitute for professional medical advice, diagnosis, or treatment.
Ozempic Important Safety Information: Read more about serious warnings and safety info.
Wegovy Important Safety Information: Read more about serious warnings and safety info.
GLP-1 Important Safety Information: Read more about serious warnings and safety info.
References
Aroda, V. R., Rosenstock, J., Terauchi, Y., et al. (2019). PIONEER 1: Randomized Clinical Trial of the Efficacy and Safety of Oral Semaglutide Monotherapy in Comparison With Placebo in Patients With Type 2 Diabetes. Diabetes Care, 42(9), 1724–1732. doi: 10.2337/dc19-0749. Retrieved from https://diabetesjournals.org/care/article/42/9/1724/36289/PIONEER-1-Randomized-Clinical-Trial-of-the
Centers for Medicare & Medicaid Services (CMS). (n.d.). Medicaid Can Cover Obesity-Related Services, Helping Beneficiaries Reduce the Risk of Chronic Disease. Retrieved from https://www.medicaid.gov/about-us/program-history/medicaid-50th-anniversary/entry/47693
Chao, A. M., Tronieri, J. S., Amaro, A., & Wadden, T. A. (2023). Semaglutide for the treatment of obesity. Trends in Cardiovascular Medicine, 33(3), 159–166. doi: 10.1016/j.tcm.2021.12.008. Retrieved from https://pubmed.ncbi.nlm.nih.gov/34942372/
Kapitza, C., Nosek, L., Jensen, L., et al. (2015). Semaglutide, a once-weekly human GLP-1 analog, does not reduce the bioavailability of the combined oral contraceptive, ethinylestradiol/levonorgestrel. Journal of Clinical Pharmacology, 55(5), 497–504. doi: 10.1002/jcph.443. Retrieved from https://pubmed.ncbi.nlm.nih.gov/25475122/
Knop, F. K., Aroda, V. R., do Vale, R. D., et al. (2023). Oral semaglutide 50 mg taken once per day in adults with overweight or obesity (OASIS 1): a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet, 402(10403), 705-719. Retrieved from https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(23)01185-6
Kommu, S. & Whitfield, P. (2024). Semaglutide. StatPearls. Retrieved on Dec. 23, 2024 from https://www.ncbi.nlm.nih.gov/books/NBK603723/
Leonard, N. (2024). “Greater Philly’s largest health insurer to restrict coverage for weight loss medications, citing ‘exorbitant costs.’” WHYY. Retrieved on Feb. 3, 2025 from https://whyy.org/articles/independence-blue-cross-restrict-coverage-ozempic-weight-loss
Novo Nordisk. (2025). Ozempic; semaglutide injection. U.S. Food and Drug Administration (FDA). Retrieved from https://www.novo-pi.com/ozempic.pdf
Novo Nordisk. (2025). Wegovy; semaglutide injection. U.S. Food and Drug Administration (FDA). Retrieved from https://www.novo-pi.com/wegovy.pdf
U.S. Food & Drug Administration (FDA-a). (2024). Compounding when drugs are on FDA’s drug shortages list. Retrieved on Dec. 23, 2024 from https://www.fda.gov/drugs/human-drug-compounding/compounding-when-drugs-are-fdas-drug-shortages-list
U.S. Food & Drug Administration (FDA-b). (2024). FDA’s concerns with unapproved GLP-1 drugs used for weight loss. Retrieved on Dec. 23, 2024 from https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/fdas-concerns-unapproved-glp-1-drugs-used-weight-loss
U.S. Food & Drug Administration (FDA-c). (2024). FDA to compounders: know your bulks and excipients suppliers. Retrieved on Dec. 23, 2024 from https://www.fda.gov/drugs/human-drug-compounding/fda-compounders-know-your-bulks-and-excipients-suppliers
U.S. Food & Drug Administration (FDA-d). (2025). FDA Approves Treatment for Serious Liver Disease Known as ‘MASH.’ Retrieved on Sep. 21, 2025 from https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-treatment-serious-liver-disease-known-mash
Wilding, J. P. H., Batterham, R. L., Davies, M., et al. (2022). Weight regain and cardiometabolic effects after withdrawal of semaglutide: The STEP 1 trial extension. Diabetes, Obesity & Metabolism, 24(8), 1553–1564. doi: 10.1111/dom.14725 Retrieved from https://pmc.ncbi.nlm.nih.gov/articles/PMC9542252/
Young, G. M., Bansal, K., Riello, R. J., 3rd, et al. (2024). Medicare coverage and patient out-of-pocket costs for cardiovascular-kidney-metabolic medications. JAMA Network Open, 7(5), e2412437. doi: 10.1001/jamanetworkopen.2024.12437. Retrieved from https://pmc.ncbi.nlm.nih.gov/articles/PMC11109768/
Zhang, P. & Patel, P. (2023). Practitioners And Prescriptive Authority. StatPearls. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK574557/
Garvey, W. T., Batterham, R. L., Bhatta, M., Buscemi, S., Christensen, L. N., Frias, J. P., Jódar, E., Kandler, K., Rigas, G., Wadden, T. A., Wharton, S., & STEP 5 Study Group (2022). Two-year effects of semaglutide in adults with overweight or obesity: the STEP 5 trial. Nature Medicine, 28(10), 2083–2091. https://www.nature.com/articles/s41591-022-02026-4